Lithium iodide
Synonym(s):Lithium iodide;Lithium monoiodide
- CAS NO.:10377-51-2
- Empirical Formula: ILi
- Molecular Weight: 133.85
- MDL number: MFCD00011092
- EINECS: 233-822-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-04-29 17:45:46
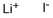
What is Lithium iodide?
Chemical properties
White to off-white crystalline powder
Physical properties
White cubic crystals; refractive index 1.955; density 4.076 g/cm3; melts at 449°C; vaporizes around 1,180°C; highly soluble in water (165 g/100g at 20°C), solubility greatly increases in hot water (433g/100g at 80°C); also very soluble in methanol (343 g/100g at 20°C) and ammonia; soluble in acetone (42.6g/100g at 18°C).
The trihydrate, LiI?3H2O, is a yellowish solid (due to the release of iodine when exposed to air); hexagonal crystals; hygroscopic; density 3.48 g/cm3; loses iodine when heated in air; loses one molecule of water of crystallization at 73°C becoming dihydrate, LiI?2H2O and loses the second molecule at 80°C, forming monohydrate, LiI?H2O and becomes anhydrous at 130°C; highly soluble in water; soluble in ethanol and acetone.
The Uses of Lithium iodide
It is used as a absorbent in refrigeration process, as a catalyst, and in photography. It is also useful for organic synthesis, pessat iodination, cleavage of esters, ethers, epoxides, decarboethoxylation, carbonylation, and aldol condensation. It is used for manufacturing medicines and mineral waters. It is used in electochemicstry and redox reactions. Lithium iodide is also a catalyzed conjugate addition reaction of β-dicarbonyl compounds. It is also useful for tetrathioquinodimethane.
The Uses of Lithium iodide
Controls regioselectivity in palladium catalyzed allylic alkylation reactions.1
The Uses of Lithium iodide
Controls regioselectivity in palladium-catalyzed allylic alkylation reactions.
What are the applications of Application
Lithium iodide, anhydrous is a reagent used for ester cleavage and carboxylation
What are the applications of Application
Lithium iodide is a reagent used for ester cleavage and carboxylation
Preparation
The trihydrate salt is obtained by neutralization of lithium hydroxide or lithium carbonate solution with pure hydriodic acid followed by concentration of the solution for crystallization:
LiOH + HI → LiI + H2O
When heated in a vacuum, the trihydrate dehydrates to anhydrous salt.
Purification Methods
Crystallise it from hot water (0.5mL/g) by cooling in a CaCl2-ice EtOH or from an acetone-Dry-Ice bath. Dry it under a vacuum over P2O5 for 1hour at 60o and then at 120o. It is deliquescent and should be stored in a tightly stoppered vessel in the dark.
Properties of Lithium iodide
| Melting point: | 446 °C(lit.) |
| Boiling point: | 1171 °C |
| Density | 3.49 g/mL at 25 °C(lit.) |
| refractive index | 1.955 |
| Flash point: | 1170-1190°C |
| storage temp. | Store below +30°C. |
| solubility | 1640g/l soluble |
| form | powder |
| appearance | White crystalline solid |
| Specific Gravity | 4.076 |
| color | White to yellow to beige |
| Water Solubility | Soluble in water, ethanol and acetone. |
| Sensitive | Hygroscopic |
| Merck | 14,5535 |
| Exposure limits | ACGIH: TWA 0.01 ppm |
| Stability: | hygroscopic |
| CAS DataBase Reference | 10377-51-2(CAS DataBase Reference) |
| EPA Substance Registry System | Lithium iodide (10377-51-2) |
Safety information for Lithium iodide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P321:Specific treatment (see … on this label). P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Lithium iodide
Lithium iodide manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
 Lithium iodide, Ultra dry CAS 10377-51-2View Details
Lithium iodide, Ultra dry CAS 10377-51-2View Details
10377-51-2 -
 Lithium iodide, Ultra dry CAS 10377-51-2View Details
Lithium iodide, Ultra dry CAS 10377-51-2View Details
10377-51-2 -
 Lithium iodide, Ultra dry CAS 10377-51-2View Details
Lithium iodide, Ultra dry CAS 10377-51-2View Details
10377-51-2 -
 Lithium iodide, Anhydrous CAS 10377-51-2View Details
Lithium iodide, Anhydrous CAS 10377-51-2View Details
10377-51-2 -
 Lithium iodide, Anhydrous CAS 10377-51-2View Details
Lithium iodide, Anhydrous CAS 10377-51-2View Details
10377-51-2 -
 Lithium iodide, Anhydrous CAS 10377-51-2View Details
Lithium iodide, Anhydrous CAS 10377-51-2View Details
10377-51-2 -
 Lithium iodide, Anhydrous CAS 10377-51-2View Details
Lithium iodide, Anhydrous CAS 10377-51-2View Details
10377-51-2 -
 Lithium iodide, Anhydrous CAS 10377-51-2View Details
Lithium iodide, Anhydrous CAS 10377-51-2View Details
10377-51-2
