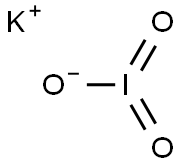
Potassium iodate
Synonym(s):Potassium iodate;Potassium iodine oxide (KIO3 )
- CAS NO.:7758-05-6
- Empirical Formula: IKO3
- Molecular Weight: 214
- MDL number: MFCD00011406
- EINECS: 231-831-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-02-18 16:13:28
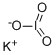
What is Potassium iodate ?
Description
Potassium iodate is an iodine-rich salt with the formula KIO3. In hot and humid climates, iodine vapor is hydrolyzed due to the hygroscopicity of potassium iodide. Compared to potassium iodide, potassium iodate is more stable and has a longer shelf life. It is a potent blocker of radioactive iodine uptake by the thyroid gland.
Chemical properties
white crystals or powder
Physical properties
Colorless crystals or white powder; monoclinic structure; density 3.90 g/cm3; stable at ordinary temperatures; melts at 560°C with partial decompo-sition, releasing oxygen; moderately soluble in cold water; 4.74 g/100mL at 0°C; greater solubility in boiling water 32.3 g/100mL at 100°C; soluble in potassium iodide solution; insoluble in alcohol and liquid ammonia.
The Uses of Potassium iodate
Potassium Iodate is a source of iodine made by reacting iodine with potassium hydroxide. it is a crystalline powder which is more stable than iodide. it has a solubility of 1 g in 15 ml of water. it is used as a fast-acting dough improver; it is used with potassium bromate as an oxidizing agent to modify the protein in bread flour which pro- motes loaf volume and shape. it is used in baked goods.
The Uses of Potassium iodate
Oxidizer used recently to form 2-styrylchromones from o-hydroxy-ω-cinnamylideneacetophenones by oxidative cyclization.1
The Uses of Potassium iodate
Oxidizing agent in volumetric chemical analysis; as maturing agent and dough conditioner.
Definition
A white solid formed either by adding iodine to a hot concentrated solution of potassium hydroxide or by the electrolysis of potassium iodide solution. No hydrates are known. It is a source of iodide and iodic acid. When treated with a dilute acid and a reducing agent, the iodate ions are reduced to iodine.
Definition
potassium iodate: A white crystallinesolid, KIO3, soluble in waterand insoluble in ethanol; monoclinic;r.d. 3.9; m.p. 560°C. It may be preparedby the reaction of iodine withhot concentrated potassium hydroxideor by careful electrolysis ofpotassium iodide solution. It is anoxidizing agent and is used as ananalytical reagent. Some potassiumiodate is used as a food additive.
What are the applications of Application
Potassium iodate is a fairly strong oxidizing agent that may be used in the assay of a number of pharmaceutical substances, for instance : benzalkonium chloride, cetrimide, hydralazine hydrochloride, potassium iodide, phenylhydrazine hydrochloride, semicarbazide hydrochloride and the like. Under appropriate experimental parameters the iodate reacts quantitatively with both iodides and iodine. It is, however, interesting to observe here that the iodate titrations may be carried out effectively in the presence of saturated organic acids, alcohol and a host of other organic substances.
The oxidation-reduction methods with potassium iodate invariably based on the formation of iodine monochloride (ICl) in a medium of strong hydrochloric acid solution.
What are the applications of Application
Potassium iodate is an oxidizing agent used in volumetric chemical analysis
Preparation
Potassium iodate can be produced by fusing potassium iodide with potassium chlorate, bromate or perchlorate:
KI + KClO3→KIO3+ KCl
The melt is extracted with water and potassium iodate is isolated from solution by crystallization.
Production Methods
Potassium iodate is formed (1) by electrolysis of potassium iodide under proper conditions, (2) by reaction of iodine and potassium hydroxide solution, and the fractional crystallization of iodate from iodide. Used as a source of iodate and iodic acid.
General Description
KIO3 can be used as a substitute of KI in radiation protection. A kinetic study of thermal degradation of KIO3 by γ-rays suggests that rate of decomposition increases while activation energy decreases upon irradiation.
Flammability and Explosibility
Non flammable
Food additive
Iodine can be added to salt in the form of potassium iodide (KI) or potassium iodate (KIO3). Because KIO3 has higher stability in the presence of salt impurities, humidity, and porous packaging, it is the recommended form.
Potassium iodate, which is formed as a secondary product, is reduced by activated carbon. The product is purified by crystallization from water. Alternatively, iron (II) iodide, prepared by using iron powder and iodine, can be treated with potassium carbonate to obtain potassium iodide. High-purity potassium iodide can be prepared by the reaction of a potassium bicarbonate with hydriodic acid.
Safety Profile
Poison by ingestion and intraperitoneal routes. A trace mineral added to animal feeds. Potentially explosive reaction with charcoal + ozone, metals (e.g., powdered aluminum, copper), arsenic carbon, phosphorus, sulfur, alkali metal hydrides, alkaline earth metal hydrides, antimony sulfide, arsenic sulfide, copper sulfide, tin sulfide, metal cyanides, metal thiocyanates, manganese dioxide, phosphorus. Violent reaction with organic matter. When heated to decomposition it emits very toxic fumes of I and K2O. See also IODATES.
Overdosage
Overdose of potassium iodate, an iodized salt used for iodine supplementation in areas endemic for goiter, has been shown to cause profound visual loss and extensive retinal pigmentary abnormalities.78 FA reveals RPE window defects and ERG and VEP testing show marked impairment of retinal function. Visual acuity may improve slowly over several months.
Purification Methods
It has been crystallised twice from distilled water (3mL/g) between 100o and 0o, dried for 2hours at 140o and cooled in a desiccator. Analytical reagent grade material dried in this way is suitable for use as an analytical standard.
Properties of Potassium iodate
| Melting point: | 560 °C(lit.) |
| Density | 3.93 g/mL at 25 °C(lit.) |
| vapor pressure | 0-0Pa at 25℃ |
| storage temp. | Store at +5°C to +30°C. |
| solubility | H2O: 0.1 M at 20 °C, clear, colorless |
| form | Powder/Solid |
| pka | 0.047[at 20 ℃] |
| color | White to off-white |
| PH | 6 (50g/l, H2O, 20℃) |
| Odor | at 100.00?%. odorless |
| Water Solubility | Soluble |
| Merck | 14,7642 |
| Solubility Product Constant (Ksp) | pKsp: 3.43 |
| Stability: | Stable. Materials to avoid include organics, combustibles, phosphorus, sulfur, carbon, powdered metals, cyanides, hydrides, strong reducing agents, aluminium, peroxides. Explosive when mixed with combustible material. |
| CAS DataBase Reference | 7758-05-6(CAS DataBase Reference) |
| EPA Substance Registry System | Potassium iodate (7758-05-6) |
Safety information for Potassium iodate
| Signal word | Danger |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H302:Acute toxicity,oral H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P220:Keep/Store away from clothing/…/combustible materials. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Potassium iodate
| InChIKey | JLKDVMWYMMLWTI-UHFFFAOYSA-M |
Potassium iodate manufacturer
DNS Fine Chemicals and Laboratories (P) Ltd
Lanya Chem Industries Pvt Ltd
Nutracare International
G Amphray Laboratories
ARRAKIS INDUSTRIES LLP
New Products
1-Amino-1-cyclohexanecarboxylic acid 6-Bromo-3-iodo-1-methyl-1H-indazole 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione S-Methylisothiosemicarbazide hydroiodide ELECTROLYTIC IRON POWDER 1-Aminocyclobutanecarboxylic acid 1-(2-Ethoxyethyl)-2-(piperidin-4-yl)-1H-benzo[d]imidazole hydrochloride tert-butyl 4-(1H-benzo[d]iMidazol-2-yl)piperidine-1-carboxylate Decanonitrile N,N'-diallyl-1,3-diaminopropanedihydrochloride N-(3-Nitrophenyl)cyclopropanecarboxamide (2-amino-2-phenylethyl)(methyl)amine Methyl-2-acetamidobenzoate methyl 6-chloro-2-(chloromethyl)nicotinate 2-methoxy-4-methyl-5-nitro pyridine 2-iodo-5-bromo pyridine 2-amino-4-methyl-5-nitro pyridine 4-cyano benzaldehyde 2-hydroxy-5-bromo pyridine 5-Fluoro-2-Oxindole 3,5-Dichloro-2,6-Dimethyl-1h-Pyridin-4-One (9H-fluoren-9-yl)methyl carbonochloridate 2-methyl-5-nitroaniline (S)-1-(tert-butoxycarbonyl)-4-oxopyrrolidine-2-carboxylic acidRelated products of tetrahydrofuran

You may like
-
 Potassium iodate 99%View Details
Potassium iodate 99%View Details -
 Potassium iodate 99%View Details
Potassium iodate 99%View Details -
 Potassium iodate, For analysis ACS CAS 7758-05-6View Details
Potassium iodate, For analysis ACS CAS 7758-05-6View Details
7758-05-6 -
 Potassium iodate, Standardized Solution CAS 7758-05-6View Details
Potassium iodate, Standardized Solution CAS 7758-05-6View Details
7758-05-6 -
 Potassium iodate CASView Details
Potassium iodate CASView Details -
 POTASSIUM IODATE 99%View Details
POTASSIUM IODATE 99%View Details -
 POTASSIUM IODATE 0.05 M (0.3N) STANDARDIZED SOLUTION traceable to NIST CASView Details
POTASSIUM IODATE 0.05 M (0.3N) STANDARDIZED SOLUTION traceable to NIST CASView Details -
 POTASSIUM IODATE 0.01667M (0.1N) STANDARDIZED SOLUTION traceable to NIST CASView Details
POTASSIUM IODATE 0.01667M (0.1N) STANDARDIZED SOLUTION traceable to NIST CASView Details