Cuprous iodide
Synonym(s):Copper monoidide;Copper(I) iodide;Cuprous iodide
- CAS NO.:7681-65-4
- Empirical Formula: CuI
- Molecular Weight: 190.45
- MDL number: MFCD00010978
- EINECS: 231-674-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:43
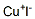
What is Cuprous iodide?
Description
Pure copper(I) iodide (CuI) is a white powder. However, samples of copper(I) iodide typically will be tan in color due to the presence of impurities.
Chemical properties
Copper(I) iodide is naturally occurring as the white to reddishbrown mineral marshite. It is essentially insoluble in water but dissolves in complexing media such as ammonia, cyanide, and halide solutions. Copper(I) iodide is manufactured pyrometallurgically by the reaction of hot copper with iodine vapor in a process essentially identical to that for the preparation of copper(I) chloride. It has also been produced by the reaction of copper powder with methanol solutions of iodine at ambient temperatures. Copper(I) iodide is used as a feed additive and as a heat and light stabilizer in certain polymers and photographic emulsions. It is also used in cloud seeding and in the oil-drilling industry as an anticorrosion adjuvant.
Chemical properties
Cuprous Iodide, Cu2O, cubic white crystals, practically insoluble in H2O or alcohol, soluble in NH4OH, potassium iodide, or potassium cyanide. Used in Sandmeyer’s reaction to synthesize aryl chlorides.
Chemical properties
Copper(I) iodide is white, but samples often appear tan or even, when found in nature as rare mineral marshite, reddish brown, but such color is due to the presence of impurities.
The Uses of Cuprous iodide
Copper (I) Iodide used in the preparation of alkynyl imines and the synthesis of pyrroles and pyrrole heterocycles. It is also used in the synthesis of BTBT derivatives ([1]Benzothieno[3,2-b]benzothio phene) for use as semiconductors in transistors.
The Uses of Cuprous iodide
A catalyst for N-arylation of amines and amino acids
The Uses of Cuprous iodide
As catalyst in organic reactions; as ice-nucleating chemical; as coating in cathode-ray tubes; as source of iodine in animal feeds; with HgI2 as tempereture indicator; bactericide.
Preparation
Copper(I) iodide can be prepared by heating iodine and copper in concentrated hydriodic acid.
In the laboratory however, copper(I) iodide is prepared by simply mixing an aqueous solution of potassium iodide and a soluble copper(II) salt such as copper(II) sulfate.
Cu2+ + 2I-→ CuI + 0.5 I2
General Description
Odorless tan or off-white solid. Sinks in water. 
Marshite, CuI, octahedral crystals to 4 mm
Reactivity Profile
Cuprous iodide has weak oxidizing or reducing powers. Redox reactions can however still occur. The majority of compounds in this class are slightly soluble or insoluble in water. If soluble in water, then the solutions are usually neither strongly acidic nor strongly basic. These compounds are not water-reactive.
Hazard
Toxic.
Health Hazard
Inhalation causes irritation of nose and throat. Ingestion of copper salts produces violent vomiting and purging, intense pain, collapse, coma, convulsions, and paralysis. Contact with eyes or skin causes irritation.
Fire Hazard
Special Hazards of Combustion Products: Irritating hydrogen iodide or iodine vapors may form in fire.
Purification Methods
It can be freshly prepared by dissolving an appropriate quantity of CuI in boiling saturated aqueous NaI over 30minutes. Pure CuI is obtained by cooling and diluting the solution with water, followed by filtering and washing sequentially with H2O, EtOH, EtOAc, Et2O and pentane, then drying in vacuo for 24hours [Dieter, J Am Chem Soc 107 4679 1985]. Alternatively wash it with H2O, then EtOH and finally with Et2O containing a little iodine. Traces of H2O are best removed first by heating at 110o and then at 400o. Exess of I2 is removed completely at 400o. It dissolves in Et2O if an amine is present to form the amine complex. On heating it becomes red, then black, but changes to white on cooling. It is sparingly soluble in H2O or alkali iodide solutions but readily soluble in NH3 (which absorbs CO) and in cyanide or thiosulfate solutions. [Glemser & Sauer in Handbook of Preparative Inorganic Chemistry (Ed Brauer) Academic Press Vol II p 1007 1965, Bawn & Ledwith Chem Ind (London) 1180 1957.]
Properties of Cuprous iodide
| Melting point: | 605 °C (lit.) |
| Boiling point: | 1290 °C/1 atm (decomp)(lit.) |
| Density | 5.62 g/mL at 25 °C (lit.) |
| vapor pressure | 10 mm Hg ( 656 °C) |
| refractive index | 2.346 |
| Flash point: | 1290°C |
| storage temp. | Store below +30°C. |
| solubility | dilute aqueous acid: insoluble(lit.) |
| appearance | White powder |
| form | Powder |
| color | White to Pale Brown |
| Specific Gravity | 5.62 |
| Water Solubility | INSOLUBLE |
| Sensitive | Air & Moisture Sensitive |
| Crystal Structure | Cubic, Sphalerite Structure - Space Group F(-4)3m |
| Merck | 14,2662 |
| Solubility Product Constant (Ksp) | pKsp: 11.9 |
| Exposure limits | ACGIH: TWA 1 mg/m3; TWA 0.01 ppm NIOSH: IDLH 100 mg/m3; TWA 1 mg/m3 |
| CAS DataBase Reference | 7681-65-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Copper(I) iodide(7681-65-4) |
| EPA Substance Registry System | Cuprous iodide (7681-65-4) |
Safety information for Cuprous iodide
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H317:Sensitisation, Skin H318:Serious eye damage/eye irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for Cuprous iodide
| InChIKey | LSXDOTMGLUJQCM-UHFFFAOYSA-M |
Cuprous iodide manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Copper(I) iodide 99%View Details
Copper(I) iodide 99%View Details -
 Copper(I) iodide CAS 7681-65-4View Details
Copper(I) iodide CAS 7681-65-4View Details
7681-65-4 -
 Copper(I) iodide CAS 7681-65-4View Details
Copper(I) iodide CAS 7681-65-4View Details
7681-65-4 -
 Copper(I) iodide CAS 7681-65-4View Details
Copper(I) iodide CAS 7681-65-4View Details
7681-65-4 -
 Copper(I) Iodide CAS 7681-65-4View Details
Copper(I) Iodide CAS 7681-65-4View Details
7681-65-4 -
 Copper(I) iodide CAS 7681-65-4View Details
Copper(I) iodide CAS 7681-65-4View Details
7681-65-4 -
 Cuprous Iodide extrapure AR CAS 7681-65-4View Details
Cuprous Iodide extrapure AR CAS 7681-65-4View Details
7681-65-4 -
 Copper (I) iodide CAS 7681-65-4View Details
Copper (I) iodide CAS 7681-65-4View Details
7681-65-4
