Nessler's Reagent
Synonym(s):Potassium tetraiodomercurate – Potassium hydroxide solution
- CAS NO.:7783-33-7
- Empirical Formula: HgI4K2
- Molecular Weight: 786.4
- MDL number: MFCD00049656
- EINECS: 231-990-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
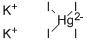
What is Nessler's Reagent?
Chemical properties
light yellow liquid
The Uses of Nessler's Reagent
Detecting the presence of ammonia, particularly in very small amounts.
The Uses of Nessler's Reagent
Mercury(II) potassium iodide is used as a pharmaceutical intermediate. It is also used as reagents, chemical research and organic intermediates.
Definition
Nessler's Reagent is a periodometallate salt that is solution of mercuric iodide in potassium iodide. It contains a tetraiodomercurate(2-).
Preparation
It is prepared by mixing 2g potassium iodide in 5ml water. To this solution, 3g of mercury (II) iodide is added, and the resulting solution is made to 20ml. Finally, 40g potassium hydroxide (30 %) is added to provide the alkaline base.
General Description
Mercuric potassium iodide, [solid] appears as odorless yellow crystals. Crystallizes with either one, two or three molecules of water. Soluble in water and denser than water. Commercially available in the anhydrous form. Highly toxic by ingestion, inhalation, and skin absorption.
Air & Water Reactions
Deliquescent. Very water soluble.
Reactivity Profile
MERCURIC POTASSIUM IODIDE is incompatible with acetylene, ammonia, chlorine dioxide, azides, calcium (amalgam formation), sodium carbide, lithium, rubidium, copper . NESSLER'S REAGENT behave in aqueous solution as a weak acid.
Hazard
Highly toxic by ingestion, inhalation, and skin absorption.
Health Hazard
Highly toxic, may be fatal if inhaled, swallowed or absorbed through skin. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Containers may explode when heated. Runoff may pollute waterways.
Safety Profile
A poison. Moderately toxic by skin contact and intraperitoneal routes. When heated to decomposition it emits very toxic fumes of Hg, K2O, and I-. See also MERCURY.
Properties of Nessler's Reagent
| Melting point: | 120-127 °C(lit.) |
| Boiling point: | 105°C |
| Density | 1.16 g/mL at 20 °C |
| Flash point: | >230 °F |
| solubility | very soluble in H2O; soluble in ethanol,ethyl ether, acetone |
| form | Liquid |
| color | Yellow to orange |
| Specific Gravity | 1.097 |
| Odor | Odorless |
| Water Solubility | Soluble in water. |
| Sensitive | Hygroscopic |
| Merck | 13,7773 |
| Exposure limits | ACGIH: TWA 0.025 mg/m3 (Skin) NIOSH: IDLH 10 mg/m3; TWA 0.05 mg/m3; Ceiling 0.1 mg/m3 |
| Stability: | Stable, but light sensitive. Incompatible with strong oxidizing agents. |
| EPA Substance Registry System | Potassium mercuric iodide (7783-33-7) |
Safety information for Nessler's Reagent
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H290:Corrosive to Metals H310:Acute toxicity,dermal H314:Skin corrosion/irritation H373:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P314:Get medical advice/attention if you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Nessler's Reagent
| InChIKey | OPCMAZHMYZRPID-UHFFFAOYSA-J |
Nessler's Reagent manufacturer
JSK Chemicals
ARRAKIS INDUSTRIES LLP
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 Mercury(II) potassium iodide CAS 7783-33-7View Details
Mercury(II) potassium iodide CAS 7783-33-7View Details
7783-33-7 -
 Mercury(II) potassium iodide CAS 7783-33-7View Details
Mercury(II) potassium iodide CAS 7783-33-7View Details
7783-33-7 -
 Nesslers reagent CAS 7783-33-7View Details
Nesslers reagent CAS 7783-33-7View Details
7783-33-7 -
 Potassium mercuric iodide CAS 7783-33-7View Details
Potassium mercuric iodide CAS 7783-33-7View Details
7783-33-7 -
 Potassium mercuric iodide, solution CAS 7783-33-7View Details
Potassium mercuric iodide, solution CAS 7783-33-7View Details
7783-33-7 -
 Nessler's (King) reagent CAS 7783-33-7View Details
Nessler's (King) reagent CAS 7783-33-7View Details
7783-33-7 -
 POTASSIUM MERCURIC IODIDE CAS 7783-33-7View Details
POTASSIUM MERCURIC IODIDE CAS 7783-33-7View Details
7783-33-7 -
 Nessler’s reagent CAS 7783-33-7View Details
Nessler’s reagent CAS 7783-33-7View Details
7783-33-7