Sodium hydrogen difluoride
Synonym(s):Sodium acid fluoride;Sodium bifluoride
- CAS NO.:1333-83-1
- Empirical Formula: F2HNa
- Molecular Weight: 61.99
- MDL number: MFCD00003529
- EINECS: 215-608-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
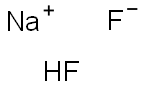
What is Sodium hydrogen difluoride?
Chemical properties
White, crystalline powder.Sol- uble in water; decomposes on heating.
The Uses of Sodium hydrogen difluoride
As a "sour" in laundering.
The Uses of Sodium hydrogen difluoride
Starting material for solid state preparation of Na3MnF6 for further high pressure structural determination studies.
General Description
Sodium bifluoride is a white crystalline solid. Sodium hydrogen difluoride is soluble in water. Sodium hydrogen difluoride is corrosive to tissue. Sodium hydrogen difluoride is used as a preservative for anatomical and zoological specimens, in metal plating and for many other uses.
Air & Water Reactions
Water soluble. Reacts with water liberating heat and forming a corrosive solution. The reaction is not violent.
Reactivity Profile
Acidic salts, such as SODIUM BIFLUORIDE, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.
Hazard
Highly toxic, strong irritant to tissue.
Health Hazard
INHALATION OF DUST: Irritating and possibly corrosive to mucous membranes. EYES: Irritating. INGESTION: Salty or soapy taste, salivation, nausea, burning or crampy abdominal pain, vomiting, diarrhea, muscle weakness, tremors. Rare: transient epileptiform convulsions, followed by CNS depression. Shock.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Flammability and Explosibility
Non flammable
Safety Profile
This material is very toxic to humans by ingestion; between 1 teaspoonful and 1 ounce may be fatal. Inhalation of dust may cause irritation to respiratory tract. Skin contact may result in irritation and ulceration; eye contact may cause burns. To fight fire, use water, foam, CO2, dry chemicals. When heated to decomposition it emits toxic fumes of Fand Na2O. See also FLUORIDES and HYDROFLUORIC ACID.
Properties of Sodium hydrogen difluoride
| Melting point: | >68 °C (dec.) |
| Boiling point: | 270℃[at 101 325 Pa] |
| Density | 2.08 |
| vapor pressure | 0.01Pa at 20℃ |
| form | pellets |
| color | white hexagonal, hexane crystals, crystalline |
| Water Solubility | g/100g solution in H2O: 3.7 (20°C), 16.4 (80°C) [KIR78] |
| Merck | 13,8656 |
| CAS DataBase Reference | 1333-83-1(CAS DataBase Reference) |
| EPA Substance Registry System | Sodium bifluoride (1333-83-1) |
Safety information for Sodium hydrogen difluoride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. |
Computed Descriptors for Sodium hydrogen difluoride
Sodium hydrogen difluoride manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Sodium Bifluoride pure CAS 1333-83-1View Details
Sodium Bifluoride pure CAS 1333-83-1View Details
1333-83-1 -
 Sodium bifluoride, 98% CAS 1333-83-1View Details
Sodium bifluoride, 98% CAS 1333-83-1View Details
1333-83-1 -
 Sodium hydrogen difluoride granular CAS 1333-83-1View Details
Sodium hydrogen difluoride granular CAS 1333-83-1View Details
1333-83-1 -
 Sodium Bifluoride pure CAS 1333-83-1View Details
Sodium Bifluoride pure CAS 1333-83-1View Details
1333-83-1 -
 Sodium hydrogen difluoride CAS 1333-83-1View Details
Sodium hydrogen difluoride CAS 1333-83-1View Details
1333-83-1 -
 Sodium Bifluoride .View Details
Sodium Bifluoride .View Details
1333-83-1 -
 Sodium Bifluoride PowderView Details
Sodium Bifluoride PowderView Details
1333-83-1 -
 Sodium Bifluoride PowderView Details
Sodium Bifluoride PowderView Details
1333-83-1
