Potassium hydrogen fluoride
Synonym(s):Potassium hydrogen difluoride;Potassium bifluoride;Potassium fluoride hydrofluoride solution;Potassium monohydrogen difluoride solution;Potassium acid bifluoride solution
- CAS NO.:7789-29-9
- Empirical Formula: F2HK
- Molecular Weight: 78.1
- MDL number: MFCD00011403
- EINECS: 232-156-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
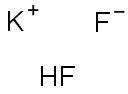
What is Potassium hydrogen fluoride?
Chemical properties
WHITE TO LIGHT GREY CRYSTALS OR CRYSTALLINE POWDER
The Uses of Potassium hydrogen fluoride
Potassium hydrogen fluoride is widely used as an etchant in glass and cleaning products. It finds an important application in the synthesis of special optical glass viz. crown and crown flint glass and as a catalyst for polymerization. It is involved in the manufacturing of wood preservatives, soldering agents and brazing. It is also used in the manufacturing of organic and inorganic fluorine compounds.
The Uses of Potassium hydrogen fluoride
In the preparation of pure potassium fluoride; as an electrolyte in the manufacture of fluorine; frosting glass; treating coal to prevent slag formation; flux for silver solders; catalyst in the alkylation of benzene with olefins.
General Description
Colorless crystals, corrosive to tissues, etches glass.
Air & Water Reactions
Water soluble giving strongly acidic solutions.
Reactivity Profile
Acidic salts, such as Potassium hydrogen fluoride, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible.
Hazard
Corrosive to tissue.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Safety Profile
A poison. Very corrosive and reactive. A corrosive irritant to the eyes, skin, and mucous membranes. When heated to decomposition it emits toxic fumes of F and K2O. See also FLUORIDES and HYDROFLUORIC ACID.
Purification Methods
It crystallises from water. It is very soluble in hot H2O and 41% at 21o. [Kwasnik in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 237 1963.]
Properties of Potassium hydrogen fluoride
| Melting point: | 239 °C(lit.) |
| Density | 2.37 g/mL at 25 °C(lit.) |
| solubility | insoluble in ethanol |
| form | Crystals or Crystalline Powder |
| color | White to light gray |
| Specific Gravity | 2.37 |
| Water Solubility | 39 g/100 mL (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,7610 |
| Exposure limits | ACGIH: TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3 |
| CAS DataBase Reference | 7789-29-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Potassium hydrogen fluoride(7789-29-9) |
| EPA Substance Registry System | Potassium bifluoride (7789-29-9) |
Safety information for Potassium hydrogen fluoride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. |
Computed Descriptors for Potassium hydrogen fluoride
Potassium hydrogen fluoride manufacturer
JSK Chemicals
Jayfluoride PVT LTD
Madras Fluorine Private Ltd
Anand Agencies
ASM Organics
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 7789-29-9 Potassium hydrogenfluoride, 98% 99%View Details
7789-29-9 Potassium hydrogenfluoride, 98% 99%View Details
7789-29-9 -
 Potassium hydrogen fluoride 98%View Details
Potassium hydrogen fluoride 98%View Details -
 Potassium hydrogen fluoride 99% CAS 7789-29-9View Details
Potassium hydrogen fluoride 99% CAS 7789-29-9View Details
7789-29-9 -
 Potassium hydrogenfluoride CAS 7789-29-9View Details
Potassium hydrogenfluoride CAS 7789-29-9View Details
7789-29-9 -
 Potassium hydrogenfluoride CAS 7789-29-9View Details
Potassium hydrogenfluoride CAS 7789-29-9View Details
7789-29-9 -
 Potassium bifluoride, 98% CAS 7789-29-9View Details
Potassium bifluoride, 98% CAS 7789-29-9View Details
7789-29-9 -
 Potassium hydrogenfluoride solution CAS 7789-29-9View Details
Potassium hydrogenfluoride solution CAS 7789-29-9View Details
7789-29-9 -
 Potassium hydrogenfluoride CAS 7789-29-9View Details
Potassium hydrogenfluoride CAS 7789-29-9View Details
7789-29-9