Hydrogen fluoride
Synonym(s):HF;Hydrofluoric acid solution, Fluoric acid;Hydrogen Fluoride
- CAS NO.:7664-39-3
- Empirical Formula: FH
- Molecular Weight: 20.01
- MDL number: MFCD00011346
- EINECS: 231-634-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 17:32:32
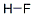
What is Hydrogen fluoride?
Description
Hydrofluoric acid is a solution of hydrogen fluoride in water. Hydrofluoric acid is highly corrosive inorganic acid. It is utilized widely in the manufacture of ceramics and graphite, in the electropolishing and pickling of metals, in the etching and frosting of glass, in the semiconductor industry as etchant and cleaning agent, in the chemical and oil-refining industries, and in cleaning solutions, laundry powder and pesticides. Hydrofluoric acid is also widely used in the preparation of many useful fluorine compounds, such as Teflon, Freon, fluorocarbons, and many medications such as fluoxetine (Prozac).
Description
Hydrogen fluoride (HF) is an extremely toxic, corrosive gas and liquid. As the lightest of the hydrogen halides, it has a surprisingly high boiling point, higher even than that of hydrogen iodide. The reason is the strong electronegativity of the fluorine atom, which causes hydrogen and fluorine atoms to form hydrogen bonds in the liquid phase.1 The other hydrogen halides are connected by much weaker van der Waals forces.
Chemical properties
colourless gas with a pungent odour
Physical properties
Hydrofluoric Acid (HF) is a solution of hydrogen fluoride in water.
While this acid is extremely corrosive and difficult to
handle, it is technically a weak acid. Hydrogen fluoride,
often in the aqueous form as hydrofluoric acid,
is a valued source of fluorine, being the precursor to
numerous pharmaceuticals such as fluoxetine (Prozac),
diverse polymers such as polytetrafluoroethylene
(Teflon), and most other synthetic materials that
contain fluorine. Hydrofluoric acid is best known to
the public for its ability to dissolve glass by reacting
with SiO2 (silicon dioxide), the major component of
most glass, to form silicon tetrafluoride gas and hexafluorosilicic acid. This property has been known
since the seventeenth century, even before hydrofluoric
acid had been prepared in large quantities by
Scheele in 1771. Because of its high reactivity toward glass, hydrofluoric acid must be stored (in small quantities) in polyethylene or Teflon containers. It is also unique in its ability to dissolve many metal and semimetal oxides. Hydrofluoric acid attacks glass by reaction with silicon dioxide to form gaseous or water-soluble silicon fluorides.
Hydrofluoric acid is very dangerous to handle,
because the human skin becomes easily saturated
with the acid. Even though skin absorption of small
areas of only 25 square inches (160 cm2) may be relatively
painless, yet the exposure may be ultimately
fatal. High concentrations of hydrofluoric acid and
hydrogen fluoride gas will also quickly destroy the
corneas of the eyes.
History
Anhydrous hydrogen fluoride was first prepared by Fremy in 1856. It mayhave been made earlier in 1670 by Schwankhard in the process of etchingglass using fluorspar and acid.
Hydrogen fluoride is the most important fluorine compound, in terms ofamounts produced and the vast number of uses. The largest application of thiscompound is in the manufacture of aluminum fluoride and sodium aluminumfluoride (cryolite) for electrolytic production of aluminum. Another majorapplication is in the manufacture of chlorofluorocarbons, which are used asrefrigerants and foaming agents; for making polymers; and for pressurizinggases. Another important application is in the processing of uranium whereHF converts uranium dioxide to uranium tetrafluoride and hexafluoride,respectively. Uranium hexafluoride is used to separate isotopes of uraniumby diffusion.
Hydrogen fluoride also is used as a catalyst in alkylation of aromatic com-pounds and for dimerization of isobutene. Other catalytic applications are inisomerization, polymerization, and dehydration reactions. Other uses are in366HYDROGEN FLUORIDEpp-03-25-new dots.qxd 10/23/02 2:38 PM Page 366 etching and polishing glasses for manufacturing light bulbs and TV tubes; inextraction of ores; in pickling stainless steel; in acidizing oil-wells; to removelaundry stains; for sample digestion in metal analysis; for removal of sandduring metal castings; as a stabilizer for rocket propellant oxidizers; and inpreparation of a number of fluoride alts of metals.
The Uses of Hydrogen fluoride
Hydrofluoric acid is used as a fluorinatingagent, as a catalyst, and in uranium refining.It is also used for etching glass and forpickling stainless steel. Hydrogen fluoridegas is produced when an inorganic fluoride is distilled with concentrated sulfuricacid.
The Uses of Hydrogen fluoride
Cleaning cast iron, copper, brass; removing efflorescence from brick and stone, or sand particles from metallic castings; working over too heavily weighted silks; frosting, etching glass and enamel; polishing crystal glass; decomposing cellulose; enameling and galvanizing iron; increasing porosity of ceramics. Its salts are used as insecticides and to arrest undesirable fermentation in brewing. Also used in analytical work to determine SiO2, etc.
The Uses of Hydrogen fluoride
Diluted hydrofluoric acid (1-3 %wt) is used in the "Oil Patch" in a mixture with other acids (HCl or organic acids) in order to stimulate the production of water, oil and gas wells specifically where sandstone is involved. HF is also used in oil refining. In a standard oil-refinery process known as Alkylation, isobutane is alkylated with low-molecular weight alkenes (primarily a mixture of propylene and butylene) in the presence of a strong acid catalyst, hydrofluoric acid. The catalyst is able to protonate the alkenes (propylene, butylene) to produce reactive carbo-cations , which cause alkylation of isobutane. The phases separate spontaneously, so the acid phase is vigorously mixed with the hydrocarbon phase to create sufficient contact surface. Hydrofluoric acid (HF) is used principally in organofluorine chemistry. Additionally, most high-volume inorganic fluoride compounds are prepared from hydrofluoric acid. Foremost are Na3AlF6, (Cryolite), and AlF3(aluminum trifluoride). A molten mixture of these solids serves as a high-temperature solvent for the production of metallic aluminum. Concerns about fluorides in the environment have led to a search for alternative technologies. Other inorganic fluorides prepared from hydrofluoric acid include NaF and UF6.
The ability of hydrofluoric acid to dissolve metal
oxides is the basis of several applications. It removes
oxide impurities from stainless steel in a process called
pickling. Surface oxides are removed from silicon with
hydrofluoric acid in the Semiconductor Industry.
For this purpose, dilute hydrofluoric acid is sold as
a household rust stain remover. Recently, it has even
been used in “Car-Wash” facilities in “wheel cleaner”
compounds.
Due to its ability to dissolve oxides, hydrofluoric acid
is useful for dissolving rock samples (usually powdered)
prior to analysis. Similarly, this acid has been used to
extract organic fossils from silicate rocks. Fossiliferous
rock may be immersed directly into the acid, or a cellulose
nitrate film may be applied (dissolved in amyl
acetate), which adheres to the organic component and
allows the rock to be dissolved around it.
Background
Hydrogen fluoride (Hydrofluoric acid) is a weak acid that is a precursor to almost all fluorine compounds, including pharmaceuticals such as Fluoxetine (Prozac). It is used as a chemical reagent in several industrial processes such as oil refinery. It is also used as etchant, cleaning agent, and a reagent to dissolve oxides and silicates.
Definition
ChEBI: A diatomic molecule containing covalently bonded hydrogen and fluorine atoms.
Definition
A colorless liquid produced by dissolving hydrogen fluoride in water. It is a weak acid, but will dissolve most silicates and hence can be used to etch glass. As the interatomic distance in HF is relatively small, the H–F bond energy is very high and hydrogen fluoride is not a good proton donor. It does, however, form hydrogen bonds.
Production Methods
Anhydrous hydrogen fluoride is manufactured by the action of sulfuric on calcium fluoride. Powdered acid-grade fluorspar (≥97% CaF2) is distilled with concentrated sulfuric acid; the gaseous hydrogen fluoride that leaves the reactor is condensed and purified by distillation.
Anhydrous hydrogen fluoride is manufactured by treating fluorspar (fluorite, CaF2) with concentrated sulfuric acid in heated kilns. The gaseous HF evolved is purified by distillation, condensed as liquid anhydrous HF, and stored in steel tanks and cylinders.
Reactions
Although anhydrous hydrogen fluoride is a very strong acid, its aqueous solution, hydrofluoric acid, is weakly acidic, particularly when dilute. The Ka value of aqueous acid at 25°C is 6.46x10–4 mol/L. It is an excellent solvent for many inorganic fluorides, forming bifluoride anion:
HF + NaF → Na+ + HF2¯ At lower temperatures (below 200°C), HF forms molecular aggregates that are held by hydrogen bonding containing linear chains of –F—H—-F—H—- F—H—-. However, above this temperature the weak hydrogen bond breaks producing monomolecular HF. Thermal dissociation of HF into elements probably occurs only at very high temperatures. Forty to 50% HF probably dissociates around 4,000°C, indicating that it is one of the most stable diatomic molecules. The most important reactions of HF involve formation of inorganic fluoridesalts. HF gas or hydrofluoric acid reacts with oxides, hydroxides, carbonates, chlorides and other metal salts forming the corresponding fluorides. Some examples are:
Bi2O3 + 6HF → 2BiF3 + 3H2O
LiOH + HF → LiF + H2O
CaCO3 + 2HF → CaF2 +CO2 + H2O
FeCl3 + 3HF → FeF3 + 3HCl
CoCl2 + 2HF → CoF2 + 2HCl Reaction with potassium dichromate yields chromyl fluoride:
K2Cr2O7 + 6HF → 2CrO2F2 + 2KF + 3H2O When ammonia gas is bubbled through a 40% ice-cold solution of hydrofluoric acid, the product is ammonium fluoride:
NH3 + HF → NH4F The addition of equimolar amount of NaOH or Na2CO3 to 40% HF instantaneously precipitates NaF:
NaOH + HF → NaF + H2O Excess HF, however, yields sodium bifluoride, NaHF2:
NaOH + 2HF → NaHF2 + H2O Reaction with phosphorus trichloride yields phosphorus trifluoride; and with phosphoryl fluoride and sulfur trioxide, the product is phosphorus pentafluoride:
PCl3 + 3HF → PF3 + HCl
POF3 + 2HF + SO3 → PF5 + H2SO4
General Description
HF is a colorless inorganic acid. Hydrogen fluoride may be formed by reacting calcium fluoride and sulfuric acid at 200oC. The fluoride in the acid has very high affinity to silicon, making it useful in etching or removal of silicon.
Air & Water Reactions
Fumes in air. Fumes are highly irritating, corrosive, and poisonous. Generates much heat on dissolution [Merck, 11th ed., 1989]. Heat can cause spattering, fuming, etc.
Reactivity Profile
Hydrofluoric acid attacks glass and any other silica containing material. May react with common metals (iron, steel) to generate flammable hydrogen gas if diluted below 65% with water. Reacts exothermically with chemical bases (examples: amines, amides, inorganic hydroxides). Can initiate polymerization in certain alkenes. Reacts with cyanide salts and compounds to release gaseous hydrogen cyanide. May generate flammable and/or toxic gases with dithiocarbamates, isocyanates, mercaptans, nitrides, nitriles, sulfides. Additional gas-generating reactions may occur with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), and carbonates. Can catalyze (increase the rate of) chemical reactions. Reacts explosively with cyanogen fluoride, methanesulfonic acid or glycerol mixed with nitric acid. Reacts violently with arsenic trioxide, phosphorus pentachloride, acetic anhydride, alkali metals, ammonium hydroxide, chlorosulfonic acid, ethylenediamine, fluorine, potassium permanganate, oleum, propylene oxide, vinyl acetate, mercury(II) oxide. Emits highly corrosive fumes of hydrogen fluoride gas when heated [Sax, 9th ed., 1996, p. 1839]. Contact with many silicon compounds and metal silicides causes violent evolution of gaseous silicon tetrafluoride [Mellor, 1956, Vol. 2, suppl. 1, p. 121].
Hazard
Toxic by ingestion and inhalation, highly corrosive to skin and mucous membranes.
Health Hazard
Hydrofluoric acid and hydrogen fluoride gasare extremely corrosive to body tissues, causing severe burns. The acid can penetrate theskin and destroy the tissues beneath and evenaffect the bones. Contact with dilute acid cancause burns, which may be perceptible hoursafter the exposure. The healing is slow. Contact with the eyes can result in impairment ofvision.
Prolonged exposure to 10–15 ppm concentrations of the gas may cause redness ofskin and irritation of the nose and eyes inhumans. Inhalation of high concentrations ofHF may produce fluorosis and pulmonaryedema. In animals, repeated exposure toHF gas within the range 20–25 ppm hasproduced injury to the lungs, liver, andkidneys.
LC50 value, inhalation (mice): 342 ppm/h.
Fire Hazard
When heated, Hydrofluoric acid emits highly corrosive fumes of fluorides. Its corrosive action on metals can result in formation of hydrogen in containers and piping to create fire hazard. Toxic and irritating vapors are generated when heated. Will attack glass, concrete, and certain metals, especially those containing silica, such as cast iron. Will attack natural rubber, leather, and many organic materials. May generate flammable hydrogen gas in contact with some metals.
Flammability and Explosibility
Hydrogen fluoride is not a combustible substance
Industrial uses
Hydrofluoric acid (HF) is a colorless liquid with a characteristic odor. It releases fumes
when in contact with moist air. Hydrofluoric acid is manufactured from fluorite containing
96–97% CaF2 by reacting it with concentrated sulfuric acid:
CaF2+H2SO4 = 2HF+CaSO4
The acid is sold as a 40% solution. The hydrofluoric acid is used as an activator and
depressant, mostly during flotation of industrial minerals (i.e. columbite, tantalite,
silica, feldspars).
Materials Uses
Carbon steel (without nonmetallic inclusions) is acceptable for handling hydrogen fluoride up to approximately 150°F (65.6°C). Aluminum- silicon-bronze, stainless steel, or nickel are suitable for cylinder valves. For higher temperatures, Monel, Inconel, nickel, or copper should be used. Cast iron or malleable fittings should be avoided. Polyethylene, lead, soft copper, Kel-F, and Teflon are acceptable gasket materials. Polyethylene, Kel-F, and Teflon are acceptable packing materials.
Potential Exposure
Mutagen;Reproductive Efector; Human Data; Primary Irritant.Hydrogen fluoride, its aqueous solution hydrofluoric acid,and its salts, are used in special metallurgical process;nuclear engineering; making organic and inorganic fluorinecompounds, such as fluorides and plastics; as a catalyst,particularly in paraffin alkylation in the petroleum industry;as an insecticide; and to arrest the fermentation in brewing.It is utilized in the fluorination processes, especially in thealuminum industry; in separating uranium isotopes; incleaning cast iron, copper, and brass; in removing efflores-cence from brick and stone; in removing sand from metalliccastings; in frosting and etching glass and enamel; in pol-ishing crystal; in decomposing cellulose; in enameling andgalvanizing iron; in working silk; in dye and analyticalchemistry; and to increase the porosity of ceramics.
Physiological effects
Hydrogen fluoride is highly corrosive to all living tissue. Contact with liquid anhydrous hydrogen fluoride, its vapor, or hydrogen fluoride solutions can cause severe bums to skin, eyes, or respiratory tract. ACGIH recommends a Threshold Limit Value-Ceiling (TLV-C) of 3 ppm (2.3 mg/m3 ) fi)r hydrogen fluoride (as F). The TLV-C is the concentration that should not be exceeded during any part of the working exposure .
First aid
If this chemical gets into the eyes, remove anycontact lenses at once (contact lenses should not be wornwhen working with HF) and irrigate immediately for atleast 30 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contacts theskin, remove contaminated clothing and flush immediatelywith large amounts of water. Immerse exposed skin area iniced 70% ethyl alcohol. Seek medical attention immedi-ately. If this chemical has been inhaled, remove from expo-sure, begin rescue breathing (using universal precautions,including resuscitation mask) if breathing has stopped andCPR if heart action has stopped. Transfer promptly to amedical facility. When this chemical has been swallowed,get medical attention. If victim is conscious, administerwater or milk. Do not induce vomiting. Medical observationis recommended for 24- 48 h after breathing overexposure,as pulmonary edema may be delayed. As first aid for pul-monary edema, a doctor or authorized paramedic may con-sider administering a corticosteroid spray.
Carcinogenicity
NTP conducted two chronic oral bioassays of fluoride administered as sodium fluoride (0, 25, 100, or 175 ppm) in drinking water for 103 weeks in rats and mice.The first study was compromised, so it was used to determine doses for the second study. NTP concluded that there was no evidence that fluoride was carcinogenic at doses up to 4.73 mg/kg/day in female rats or at doses up to 17.8 and 19.9 mg/kg/day in male and female mice, respectively.
Environmental Fate
Hydrogen fluoride is a colorless, fuming liquid with a strong,
irritating odor. The density is 1.002 at 0 ℃ and the boiling
point is 19.51 ℃. Hydrogen Fluoride is naturally released into
the environment, primarily from volcanoes, ranging from 0.6
to 6 million metric tons per year. The majority of artificial
pollutants come from electrical utilities.
Hydrogen fluoride is removed from air by wet deposition as
fluoride salts with an atmospheric lifetime of 1–5 days.
Metabolism
Not Available
Storage
All work with HF should be conducted in a fume hood to prevent exposure by inhalation, and splash goggles and neoprene gloves should be worn at all times to prevent eye and skin contact. Containers of HF should be stored in secondary containers made of polyethylene in areas separate from incompatible materials. Work with anhydrous HF should be undertaken using special equipment and only by well-trained personnel familiar with first aid procedures.
Shipping
Hydrogen fluoride, anhydrous, requires a ship-pinglabel of “CORROSIVE, POISONOUS/TOXICMATERIALS.”It falls in Hazard Class 8 and PackingGroup I.Hydrofluoric acid, with >60% strength, requires a shippinglabelof“CORROSIVE,POISONOUS/TOXICMATERIALS." It falls in Hazard Class 8 and PackingGroup I.Hydrofluoric acid, with not >60% strength, requires a ship-ping .labelof“CORROSIVE,POISONOUS/TOXICMATERIALS." It falls in Hazard Class 8 and PackingGroup II.
Purification Methods
It can be purified by trap-to-trap distillation, followed by drying over CoF2 at room temperature and further distillation. Alternatively, it can be absorbed on NaF to form NaHF2 which is then heated under vacuum at 150o to remove volatile impurities. The HF is regenerated by heating at 300o and is stored with CoF3 in a nickel vessel, being distilled as required. (Water content should be ca 0.01%.) To avoid contact with base metal, use can be made of nickel, polychlorotrifluoroethylene and gold-lined fittings [Hyman et al. J Am Chem Soc 79 3668 1957]. An aqueous solution is hydrofluoric acid (see above). It is HIGHLY TOXIC and attacks glass.
Toxicity evaluation
HFA is toxic by ingestion, inhalation, and (most commonly) by dermal exposure. It is highly corrosive to the skin and mucous membranes with very short (5 s or less) exposure concentrations of 0.003% and above, acting by protonation of tissues. It causes a liquefying necrosis at the site of contact. Absorption of fluoride ions leads to systemic fluoride poisoning, in turn leading to hypokalemia and hypomagnesemia potentially resulting in neuromuscular paralysis and cardiac arrhythmias.
Incompatibilities
HF reacts with glass, ceramics, and some metals. Reactions with metals may generate potentially explosive hydrogen gas.
Waste Disposal
Excess hydrogen fluoride and waste material containing this substance should be placed in an appropriate container, clearly labeled, and handled according to your institution's waste disposal guidelines. For more information on disposal procedures, see Chapter 7 of this volume.
References
[1] David J. Monk, and David S. Soane, A review of the chemical reaction mechanism and kinetics for hydrofluoric acid etching of silicon dioxide for surface micromachining applications, Thin Solid Films, 1993, vol. 232, 1-12
[2] P. Sanz-Gallen, S. Nogue, P. Munne and A. Faraldo, Hybocalcaemia and hypomagnesaemia due to hydrofluoric acid, Occup Med (Lond), 2001, vol. 51, 294-295
GRADES AVAILABLE
Anhydrous hydrogen fluoride is available from a number of suppliers with grades ranging from 99.0 percent to 99.96 percent. The major impurities are water (H20) and sulfur dioxide (S02).
Properties of Hydrogen fluoride
| Melting point: | -35°C |
| Boiling point: | 105°C |
| Density | 1.15 g/mL at 25 °C(lit.) |
| vapor density | 1.27 (vs air) |
| vapor pressure | 25 mm Hg ( 20 °C) |
| Flash point: | 112°C |
| storage temp. | Store at +5°C to +30°C. |
| solubility | miscible |
| solubility | very soluble in H2O, ethanol; soluble in ethyl ether |
| pka | 3.17(at 25℃) |
| appearance | colorless gas |
| form | Liquid, Double Sub-Boiling Quartz Distillation |
| color | max. 10 |
| Specific Gravity | 1.15 |
| PH Range | 1 |
| PH | 3.27(1 mM solution);2.65(10 mM solution);2.12(100 mM solution) |
| Odor | Acrid, irritating odor |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| Merck | 14,4790 |
| Dielectric constant | 17.0(-73℃) |
| Exposure limits | Ceiling limit 3 ppm (~2.5 mg/m3) as F
(ACGIH); TWA 3 ppm (MSHA and OSHA). |
| Stability: | Stable. Hygroscopic. Incompatible with glass, alkali metals, light metals, alkaline earth metals |
| CAS DataBase Reference | 7664-39-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Hydrogen fluoride(7664-39-3) |
| EPA Substance Registry System | Hydrofluoric acid (7664-39-3) |
Safety information for Hydrogen fluoride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Hydrogen fluoride
Hydrogen fluoride manufacturer
JSK Chemicals
Suvidhinath Laboratories
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran





You may like
-
 Anhydrous Hydrofluoric Acid 99%View Details
Anhydrous Hydrofluoric Acid 99%View Details -
 Hydrofluoric Acid 99%View Details
Hydrofluoric Acid 99%View Details -
 Hydrofluoric acid CAS 7664-39-3View Details
Hydrofluoric acid CAS 7664-39-3View Details
7664-39-3 -
 Hydrofluoric acid 40% CASView Details
Hydrofluoric acid 40% CASView Details -
 Hydrofluoric acid 40% CASView Details
Hydrofluoric acid 40% CASView Details -
 Hydrofluoric Acid 40, For Metal Treatment, Industrial GradeView Details
Hydrofluoric Acid 40, For Metal Treatment, Industrial GradeView Details
7664-39-3 -
 Hydrofluoric Acid (30%,40%, 60%,80%), Industrial Grade, For Glass EtchingView Details
Hydrofluoric Acid (30%,40%, 60%,80%), Industrial Grade, For Glass EtchingView Details
16872-11-0 -
 Hydrofluoric Acid 70%, Technical GradeView Details
Hydrofluoric Acid 70%, Technical GradeView Details
7664-39-3
