Sodium bromide
Synonym(s):Bromide standard for aqueous matrices ready for use;Bromo sodium;Sodium bromide;Sodium bromide solution
- CAS NO.:7647-15-6
- Empirical Formula: NaBr
- Molecular Weight: 102.89
- MDL number: MFCD00003475
- EINECS: 231-599-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-16 17:17:29
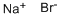
What is Sodium bromide?
Chemical properties
Sodiumbromide, NaBr,is a white, hygroscopic, crystalline solid with a bitter, saline taste.It is water soluble,with a melting point of 758°C (1400 OF). Sodium bromide is used in medicine as a sedative and in photography in the preparation of silver bromide emulsion on photographic plates or films.
Physical properties
White crystalline powder or granules; saline and slight bitter taste; cubic structure; density 3.20 g/cm3; melts at 747° C; vaporizes at 1,390°C; vapor pressure 1 torr at 806°C and 5 torr at 903°; highly soluble in methanol, 16.7 g/100mL.
The dihydrate is a white crystalline solid; density 2.18 g/cm3; decomposes at 36°C; soluble in water; sparingly soluble in methanol.
Occurrence
Sodium bromide occurs in seawater at an average concentration of 0.008%. It also is found naturally in some salt deposits. It is used in photography for preparing light-sensitive silver bromide emulsions. The salt also is used as a bleaching and disinfecting agent for water treatement in swimming pools, health spas, and hot tubs. Other uses are as a catalyst for partial oxidation of hydrocarbons, for increasing density of aqueous drillng fluids for oil wells, as an electrolyte component in sodium-halogen batteries, as a brominating agent in organic synthesis, in preparing bromide salts, and as a laboratory reagent. Sodium bromide is used in medicine as a sedative and hypnotic.
The Uses of Sodium bromide
Sodium Bromide is a high-tonnage chemical and one of the most essential bromide salts (NaBr2). It is a source of bromide ions. High-purity grades are required in the formulation of silver bromide emulsions for photography. The compound, usually in combination with hypochlorites, is used as a bleach, notably for cellulosic. The production of sodium bromide simply involves the neutralization of HBr with NaOH sodium carbonate or bicarbonate.
The Uses of Sodium bromide
Sodium Bromide is an inorganic compoiund used as a catalyst in the photoinduced polymerization of acrylates.
Definition
ChEBI: Sodium bromide is an inorganic sodium salt having bromide as the counterion. It is a bromide salt and an inorganic sodium salt. It is used in photographic processingand in analytical chemistry.
Preparation
Sodium bromide can be prepared by several methods. Pure salt can be made by neutralizing sodium hydroxide or sodium carbonate with hydrobromic acid. The solution is evaporated for crystallization:NaOH + HBr → NaBr + H2O NaCO3 + HBr → NaBr + CO2 + H2O
Sodium bromide can be made by passing bromine through an aqueous solution of sodium hydroxide or carbonate in the presence of a reducing agent, such as ammonia, hydrazine, activated charcoal, or Fe2+ ion. A typical method involves adding iron to bromine water to form ferrosoferric bromide, Fe[FeBr5]. This double salt is dissolved in excess water followed by addition of sodium carbonate. The product mixture is filtered and the filtrate is evaporated to crystallize sodium bromide. The overall reaction may be written as follows: 3Fe + 4Br2 + 4Na2CO3 → 8NaBr + FeCO3 + Fe2(CO3)3
Another method involves adding excess bromine to a solution of sodium hydroxide. This forms sodium bromide and bromate. The product solution is evapoated to dryness. The bromate is reduced to bromide by heating with carbon: 3Br2 + 2NaOH + H2O → NaBr + NaBrO3 + 4HBr.
General Description
Sodium bromide is a brominating agent mainly used in organic synthetic reactions as a bromide source.
Hazard
Toxic by inhalation and ingestion.
Flammability and Explosibility
Non flammable
Biochem/physiol Actions
Sodium bromide can be used as a substitute for potassium bromide.
Safety Profile
Moderately toxic by ingestion. Experimental reproductive effects. Incompatible with acids, alkaloidal and heavy-metal salts. When heated to decomposition it emits toxic fumes of Brand NazO. See also BROMIDES.
Description
Sodium bromide is a chemical compound that is a white crystalline solid at room temperature. The compound is inorganic, water-soluble, and has the molecular formula NaBr. Because sodium bromide is comprised of ionically bonded sodium atoms to bromine atoms, it is often used as a source of bromide ions. The compound is synthesized by combining sodium hydroxide (NaOH) with hydrogen bromide (HBr). When combined, the salts of sodium bromide crystallize around 50.7° Celsius; without water, it crystallizes above that temperature, and with water, it crystallizes below this temperature.
Chemical property
Sodium bromide is an inorganic compound in its dry form, a white crystalline powder with a salty and somewhat bitter taste. The chemical formula for sodium bromide in NaBr. It is a white crystal or white, granular powder having the odor of sulfur dioxide. It does not occur as a natural solid due to its solubility; it is extracted from ocean water along with chlorides, iodides, and halites.
Purification Methods
Crystallise the bromide from water (0.86mL/g) between 50o and 0o, and dry it at 140o under vacuum (this purification may not eliminate chloride ion).
Incompatibilities
Sodium bromide is compatible with most non-metallic materials of construction such as polypropylene, polyethylene, fiberglass reinforced plastic (FRP), cellulose, cloth, coatings, rubbers, etc. Metals can also be used provided the sodium bromide is kept dry. If the sodium bromide becomes wet, steel will suffer general corrosion and stainless steels and aluminum will suffer pitting attack. The rates of attack will depend upon the amount of oxygen present but in general will not be rapid.
Properties of Sodium bromide
| Melting point: | 755 °C (lit.) |
| Boiling point: | 1390 °C |
| Density | 3,203 g/cm3 |
| vapor pressure | 1 mm Hg ( 806 °C) |
| refractive index | 1.6412 |
| Flash point: | 1390°C |
| storage temp. | Store at room temperature. |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | Powder |
| color | White |
| Specific Gravity | 3.21 |
| PH | 5.74 (430g/l, H2O, 22.5℃) |
| Water Solubility | 905 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| λmax | λ: 260 nm Amax: 0.01 λ: 280 nm Amax: 0.01 |
| Merck | 14,8594 |
| BRN | 3587179 |
| Dielectric constant | 6.3399999999999999 |
| Stability: | Stable. Incompatible with strong acids. Hygroscopic. |
| CAS DataBase Reference | 7647-15-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Sodium bromide(7647-15-6) |
| EPA Substance Registry System | Sodium bromide (7647-15-6) |
Safety information for Sodium bromide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for Sodium bromide
| InChIKey | JHJLBTNAGRQEKS-UHFFFAOYSA-M |
Sodium bromide manufacturer
JSK Chemicals
Canton Laboratories Private Limited
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 Sodium Bromide 98%View Details
Sodium Bromide 98%View Details -
 SODIUM BROMIDE 99%View Details
SODIUM BROMIDE 99%View Details -
 Sodium bromide 99%View Details
Sodium bromide 99%View Details -
 Sodium bromide 99%View Details
Sodium bromide 99%View Details -
 Sodium bromide 98%View Details
Sodium bromide 98%View Details -
 Sodium bromide 99%View Details
Sodium bromide 99%View Details -
 sodium bromide CASView Details
sodium bromide CASView Details -
 Bromide standard solution CASView Details
Bromide standard solution CASView Details
