Calcium bromide
Synonym(s):Hydrobromic acid calcium salt
- CAS NO.:7789-41-5
- Empirical Formula: Br2Ca
- Molecular Weight: 199.89
- MDL number: MFCD00010902
- EINECS: 232-164-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-03 19:45:19
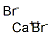
What is Calcium bromide?
Description
Calcium bromide has the chemical formula CaBr2. It
was first prepared by Pfeiffer, Klossmann and Angern
in 1924. It is a deliquescent salt that has the form of
colorless hexagonal crystals that are soluble in water and absolute alcohol. Its molecular weight is 199.90 g/
mol for the anhydride. When strongly heated in air,
calcium bromide will produce CaO (lime) and Br2. It
is generally obtained as the dihydrate, CaBr2·2H2O,
in the form of a white crystalline powder. A hexahydrate,
CaBr2·6H2O, is also known. Anhydrous calcium
bromide is obtained by dehydration (250°C, vacuum)
of the dihydrate CaBr2·2H2O. On contact with hot
surfaces or flames, CaBr2 decomposes forming
bromine as a toxic and corrosive fume. It also reacts
with strong acids to produce corrosive fumes of
bromine.
In its solid anhydrous state, it is a white powder.
CaBr2 can be found in drilling muds, neuroses medication,
freezing mixtures, food preservatives, photography
and fire retardants. The anhydride has a melting point
of 730°C and a boiling point of 810°C where it decomposes.
Its density is 3.41 g/cm3. It is moderately soluble
in water at 142 g/100 ml of water.
Chemical properties
Calciumbromide, CaBr2, is a colorless crystalline solid with a melting point of 765°C. It is deliquescent and is soluble in water and absolute alcohol. Calcium bromide is used in medicine. Calcium bromide(hydrated), CaBr2·3H20, has a meiting point of 80.5℃.
The Uses of Calcium bromide
Made by the action of hydrobromic acid on calcium oxide and crystallization. The white granular crystals are soluble in water. Calcium bromide was used in making collodion emulsions, particularly for dye-sensitized plates.
The Uses of Calcium bromide
In photography for making dry plates and light-sensitive papers; manufacture of mineral waters, NH4Br, fire-extinguishing compositions.
The Uses of Calcium bromide
Calcium bromide has a number of useful applications. Used in the oil and gas fields, the compound is a true working example. At almost every stage of the oil and gas extraction process, calcium bromide has found extensive usage. Stability, dependability and ease of use distinguish calcium bromide in the drilling process. There, it is used in “drilling muds” worldwide.
Preparation
CaBr2·2H2O has a melting point of 38°C where it
dissolves in its own waters of hydration. Its density is
2.290 g/cm3. It can be prepared by reaction in solution
with HBr on the carbonate:
CaCO3 (solid)+HBr (liq)→CaBr2 (liq)+ CO2 (gas)
An improved method for producing calcium bromide
has been developed by reacting hydrogen bromide with
calcium hydroxide in the presence of water.
Definition
ChEBI: Calcium dibromide is a calcium salt.
Properties of Calcium bromide
| Melting point: | 730°C |
| Boiling point: | 806-812°C |
| Density | 3.353 g/mL at 25 °C (lit.) |
| vapor pressure | 0Pa at 25℃ |
| Flash point: | 806-812°C |
| solubility | soluble in ethanol, acetone |
| form | beads |
| color | White to off-white |
| Specific Gravity | 3.353 |
| Odor | gran. or rhombic cryst., odorless, sharp saline taste |
| Water Solubility | Soluble in water, methanol, ethanol and acetone. Insoluble in dioxane, chloroform and ether. |
| Sensitive | Hygroscopic |
| Merck | 14,1654 |
| Stability: | hygroscopic |
| CAS DataBase Reference | 7789-41-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Calcium dibromide(7789-41-5) |
| EPA Substance Registry System | Calcium bromide (CaBr2) (7789-41-5) |
Safety information for Calcium bromide
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Calcium bromide
Calcium bromide manufacturer
JSK Chemicals
Kavya Pharma
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 CALCIUM BROMIDE 99%View Details
CALCIUM BROMIDE 99%View Details -
 Calcium bromide, Ultra dry CAS 7789-41-5View Details
Calcium bromide, Ultra dry CAS 7789-41-5View Details
7789-41-5 -
 Calcium bromide, Ultra dry CAS 7789-41-5View Details
Calcium bromide, Ultra dry CAS 7789-41-5View Details
7789-41-5 -
 Calcium Bromide PowderView Details
Calcium Bromide PowderView Details
7789-41-5 -
 Calcium Bromide Solution 52%View Details
Calcium Bromide Solution 52%View Details
7789-41-5 -
 Calcium bromide solution 52%View Details
Calcium bromide solution 52%View Details
7789-41-5 -
 Calcium Bromide Solution 52%, 340 Kgs Drums / 1700 Kgs IbcView Details
Calcium Bromide Solution 52%, 340 Kgs Drums / 1700 Kgs IbcView Details
7789-41-5 -
 Calcium Bromide PowderView Details
Calcium Bromide PowderView Details
7789-41-5
