Magnesium bromide hexahydrate
Synonym(s):Magnesium dibromide hexahydrate
- CAS NO.:13446-53-2
- Empirical Formula: Br2H2MgO
- Molecular Weight: 202.13
- MDL number: MFCD00149780
- EINECS: 603-827-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-14 14:09:35

What is Magnesium bromide hexahydrate?
Chemical properties
Magnesium Bromide Hexahydrate which crystallizes from an aqueous solution at temperatures above 0°C, is highly hygroscopic and isomorphous with MgCl2·6H2O. Magnesium Bromide has two different forms - anhydrous and hexahydrate forms. It appears as white hygroscopic crystals in the anhydrous form and as colorless monoclinic crystals in the hexahydrate form.
The Uses of Magnesium bromide hexahydrate
Magnesium bromide occurs in sea water, surface and subterranean brines, and salt deposits. It is an electrolyte component in certain dry cells. In medicine, it is a sedative and anticonvulsant for treatment of nervous disorder. It also is used in organic synthesis forming several addition compounds.
Production Methods
It is also formed by the reaction of magnesium carbonate and hydrobromic acid. The solubility of magnesium bromide is 101 g/ 100 mL of water at 20 °C; the solubility of the hexahydrate is 160 g/ 100 mL of 95% ethanol at 20 °C.
Preparation
Magnesium bromide is prepared by treating MgO
with hydrobromic acid and then crystallization
above 0.0°C in solution. The product is the hexahydrate
salt:
MgO+2HBr→MgBr2+H2O→MgBr2·6H2O
The anhydrous form may also be prepared by heating
the hexahydrate with dry HBr gas. This compound can
also be formed directly from the elements:
Mg+Br2→MgBr2
What are the applications of Application
Magnesium bromide hexahydrate is used as flame retardant. Used as source of magnesium ion and a co-factor for many enzymes including deoxyribonuclease (DNase) and various restriction enzymes.
Properties of Magnesium bromide hexahydrate
| Melting point: | 165 °C |
| Density | 2 g/mL at 25 °C(lit.) |
| storage temp. | Hygroscopic, Room Temperature, under inert atmosphere |
| solubility | Methanol, Water |
| form | Crystalline Powder |
| color | White |
| Specific Gravity | 2 |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| Stability: | Very Hygroscopic |
| CAS DataBase Reference | 13446-53-2(CAS DataBase Reference) |
Safety information for Magnesium bromide hexahydrate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Magnesium bromide hexahydrate
| InChIKey | OTCKOJUMXQWKQG-UHFFFAOYSA-L |
Abamectin manufacturer
JSK Chemicals
Rivashaa Agrotech Biopharma Pvt. Ltd.
Axiom Chemicals Pvt Ltd (Axiom Corporation)
Pallav Chemicals And Solvents Pvt Ltd
Anand Agencies
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran


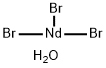
You may like
-
 Magnesium bromide hexahydrate 98%View Details
Magnesium bromide hexahydrate 98%View Details -
 Magnesium bromide hexahydrate CAS 13446-53-2View Details
Magnesium bromide hexahydrate CAS 13446-53-2View Details
13446-53-2 -
 Magnesium bromide hexahydrate CASView Details
Magnesium bromide hexahydrate CASView Details -
 Magnesium bromide hexahydrate 99% CAS 13446-53-2View Details
Magnesium bromide hexahydrate 99% CAS 13446-53-2View Details
13446-53-2 -
 Magnesium bromide CAS 13446-53-2View Details
Magnesium bromide CAS 13446-53-2View Details
13446-53-2 -
 MAGNESIUM BROMIDE HEXAHYDRATE Extra Pure CAS 13446-53-2View Details
MAGNESIUM BROMIDE HEXAHYDRATE Extra Pure CAS 13446-53-2View Details
13446-53-2 -
 Magnesium bromide hexahydrate 98.00% CAS 13446-53-2View Details
Magnesium bromide hexahydrate 98.00% CAS 13446-53-2View Details
13446-53-2 -
 Magnesium bromide hexahydrate CAS 13446-53-2View Details
Magnesium bromide hexahydrate CAS 13446-53-2View Details
13446-53-2