Cupric bromide
Synonym(s):brominating agent;Copper dibromide;Copper(II) bromide;Cupric bromide
- CAS NO.:7789-45-9
- Empirical Formula: Br2Cu
- Molecular Weight: 223.35
- MDL number: MFCD00010970
- EINECS: 232-167-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
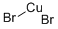
What is Cupric bromide?
Chemical properties
Black crystal
The Uses of Cupric bromide
As intensifier in photography; as brominating agent in organic synthesis; as humidity indicator; as wood preservative; in solid-electrolyte battery; as stabilizer for acetylated polyformaldehyde.
The Uses of Cupric bromide
Copper(II) bromide is used in photographic processing as an intensifier and as a brominating agent in organic synthesis. It is also used in the coupling of o-iodophenols and aryl acetylenes avoiding the use of palladium. It finds application in the preparation of NK1/NK2 receptor antagonists used in the regulation of tachykinin.
The Uses of Cupric bromide
Some reported applications of cupric bromide are:
catalyst in cross coupling reactions.
co-catalyst in Sonogashira coupling.
Lewis acid in enantioselective addition of alkynes.
reducing agent, when complexed by three molecules of pyridine initiators for the controlled polymerization of styrene, methyl acrylate and methyl methacrylate.
Reductive homocoupling of α-bromo- α- chlorocarboxylates to dimethyl α, α′ dichlorosuccinate derivatives in presence of CuBr/LiOCH3 in methanol has been reported.
General Description
Odorless black solid. Sinks and mixes with water.
Air & Water Reactions
Deliquescent. Water soluble.
Reactivity Profile
Acidic inorganic salts, such as Cupric bromide, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.
Health Hazard
Inhalation of dust causes irritation of throat and lungs. Ingestion of large amounts causes violent vomiting and purging, intense pain, collapse, coma, convulsions, and paralysis. Contact with solutions causes eye irritation; contact with solid causes severe eye surface injury and skin irritation.
Fire Hazard
Special Hazards of Combustion Products: Irritating hydrogen bromide gas may form in fire.
Flammability and Explosibility
Not classified
Synthesis
Copper(II) bromide can be obtained by combining copper oxide and hydrobromic acid:
CuO + 2HBr → CuBr2 + H2O.
Purification Methods
Crystallise it twice by dissolving it in water (140mL/g), filtering to remove any Cu2Br2, and concentrating under vacuum at 30o until crystals appear. The cupric bromide is then allowed to crystallise by leaving the solution in a vacuum desiccator containing P2O5 [Hope et al. J Chem Soc 5226 1960, Glemser & Sauer in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol II p 1009 1965].
Properties of Cupric bromide
| Melting point: | 498 °C(lit.) |
| Boiling point: | 900 °C |
| Density | 4.77 g/mL at 25 °C(lit.) |
| Flash point: | 900°C |
| storage temp. | Store below +30°C. |
| solubility | 1200g/l |
| form | Crystalline Powder |
| appearance | Grayish black crystals |
| Specific Gravity | 4.77 |
| color | Gray-blue |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| Merck | 14,2629 |
| Exposure limits | ACGIH: TWA 1 mg/m3 NIOSH: IDLH 100 mg/m3; TWA 1 mg/m3 |
| Stability: | Stable. Incompatible with alkali metals, moisture. Reacts violently with potassium. |
| CAS DataBase Reference | 7789-45-9(CAS DataBase Reference) |
| NIST Chemistry Reference | copper(II) dibromide(7789-45-9) |
| EPA Substance Registry System | Copper bromide (CuBr2) (7789-45-9) |
Safety information for Cupric bromide
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H314:Skin corrosion/irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Cupric bromide
Cupric bromide manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
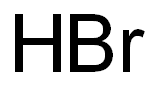


You may like
-
 Copper(II) bromide 98%View Details
Copper(II) bromide 98%View Details -
 Copper(II) bromide, Cu 28.1% min. CAS 7789-45-9View Details
Copper(II) bromide, Cu 28.1% min. CAS 7789-45-9View Details
7789-45-9 -
 Copper(II) bromide, Cu 28.1% min. CAS 7789-45-9View Details
Copper(II) bromide, Cu 28.1% min. CAS 7789-45-9View Details
7789-45-9 -
 Copper (II) bromide 98% CAS 7789-45-9View Details
Copper (II) bromide 98% CAS 7789-45-9View Details
7789-45-9 -
 Copper (II) BromideView Details
Copper (II) BromideView Details
7789-45-9 -
 25G Cupric Bromide CAS Number:7789-45-9, PowderView Details
25G Cupric Bromide CAS Number:7789-45-9, PowderView Details
7789-45-9 -
 Copper (II) Bromide, 98.5%, 500gm BottleView Details
Copper (II) Bromide, 98.5%, 500gm BottleView Details
7787-70-4 -
 Laboratory Cupric Bromide, 25Kg Bag, 98%View Details
Laboratory Cupric Bromide, 25Kg Bag, 98%View Details
7787-70-4
