Magnesium carbonate
Synonym(s):Magnesium carbonate;Magnesium carbonate hydrate
- CAS NO.:13717-00-5
- Empirical Formula: CH2O3.Mg
- Molecular Weight: 86.33
- MDL number: MFCD00064632
- EINECS: 208-915-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-16 17:17:29
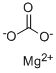
What is Magnesium carbonate?
Description
Magnesium carbonate has the molecular formula of
MgCO3 and the molecular weight of 84.3145 g/mol.For the most part,Mg2+
forms several hydrated and
basic carbonates that are stable and occur in nature.
The calcite structure of magnesium
carbonate has the form wherein Mg2+
is surrounded
by six O2-
atoms. The dihydrate composition has
a triclinic structure, while the trihydrate has a monoclinic
structure. References to “light” and “heavy” magnesium
carbonates actually refer to the magnesium hydroxycarbonates.
A space-filling structure of the anhydrous
salt is seen in the above diagram where the triangular
CO3 2-
groups are clearly visible.
Chemical properties
Light, bulky, white powder. Soluble in acids; very slightly soluble in water; insoluble in alcohol. Noncombustible.
Chemical properties
At room temperature, linolenic acid is a colorless liquid; soluble in most organic solvents; insoluble in water. Combustible.
The Uses of Magnesium carbonate
Magnesium carbonate (MgCO3) is found in a mixture of natural minerals. It can also be produced in several ways, including pumping carbon dioxide through magnesium oxide or magnesium hydroxide. It is used in pharmaceuticals such as magnesium citrate and as a desiccant to keep hydroscopic products from caking (table salt) and to strengthen rubber and produce dyes, inks, and cosmetics.
The Uses of Magnesium carbonate
Magnesium salts, heat insulation and refractory, rubber reinforcing agent, inks, glass, pharmaceuticals, dentifrice and cosmetics, free-running table salts, antacid, making magnesium citrate, filtering medium. In foods as drying agent, color retention agent, anticaking agent, carrier.
The Uses of Magnesium carbonate
Magnesium Carbonate is an anticaking agent and general purpose food additive. it is practically insoluble in water but is more soluble in water containing carbon dioxide. it imparts a slightly alkaline reaction to the water. it is used as an alkali in sour cream, butter, and canned peas. it is used as an anticaking agent in table salt and dry mixes. it assists in providing clarity in algin gels and functions as a filler in dental impression materials.
Definition
The term magnesium carbonate is generally reserved for the synthetic, pure variety. The naturally occurring material is called magnesite.
Properties of Magnesium carbonate
| Melting point: | 2200 °C |
| Density | 3.001 g/cm3 |
| storage temp. | Hygroscopic, Room Temperature, under inert atmosphere |
| form | Solid |
| color | White to Off-White |
| Solubility Product Constant (Ksp) | pKsp: 5.17 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 13717-00-5(CAS DataBase Reference) |
| EPA Substance Registry System | Magnesite (13717-00-5) |
Safety information for Magnesium carbonate
Computed Descriptors for Magnesium carbonate
Magnesium carbonate manufacturer
ASM Organics
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 Magnesium Carbonate 98%View Details
Magnesium Carbonate 98%View Details -
 MAGNESIUM CARBONATE 99%View Details
MAGNESIUM CARBONATE 99%View Details -
 Magnesium Carbonate 99%View Details
Magnesium Carbonate 99%View Details -
 Magnesium Carbonate 99%View Details
Magnesium Carbonate 99%View Details -
 Magnesium carbonate 99%View Details
Magnesium carbonate 99%View Details -
 Magnesium carbonate 98%View Details
Magnesium carbonate 98%View Details -
 magnesium carbonate, natural 13717-00-5 99%View Details
magnesium carbonate, natural 13717-00-5 99%View Details
13717-00-5 -
 13717-00-5 magnesium carbonate, natural 98%View Details
13717-00-5 magnesium carbonate, natural 98%View Details
13717-00-5
