Silver carbonate
Synonym(s):Disilver carbonate;Fetizon′s reagent;Silver(I) carbonate
- CAS NO.:534-16-7
- Empirical Formula: CAg2O3
- Molecular Weight: 275.75
- MDL number: MFCD00003403
- EINECS: 208-590-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
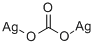
What is Silver carbonate?
Chemical properties
Silver carbonate (chemical formula: Ag2CO3) is a light yellow or yellow-green powder, slightly soluble in water, and easily turns dark purple or dark gray under long-term light. After heating, it will decompose to produce silver oxide, which is used to prepare other silver compounds.
The Uses of Silver carbonate
Silver carbonate is a mild oxidizing agent for conversion of alcohols to aldehydes and ketones. It is also used as Biological stain and Koenigs-Knorr glycosylation.
What are the applications of Application
Silver Carbonate on Celite is a reagent used in the synthesis of 3-Oxo-12a-hydroxy-5β-cholanoic Acid which is a keto bile acid derivative.
Preparation
Silver carbonate is prepared by the reaction of sodium carbonate and silver nitrate.
Reaction: Dissolve 53g of sodium carbonate in 600ml of water and slowly add it to a solution of 172g of silver nitrate dissolved in 2L of water (10min). Silver nitrate is in a slight excess. The reaction mixture was vigorously stirred with a mechanical stirrer, and the silver carbonate was filtered off, washed with a small amount of acetone to facilitate drying, and then air-dried. All operations must be carried out in a dark room.
Reactions
Silver carbonate is not a powerful oxidizing agent but it is useful in Organic chemistry. Rapoport et al. were probably the first to use silver carbonate for the oxidation of alcohols to carbonyl derivatives. Rapoport refluxed codeine with silver carbonate in benzene and obtained a 75% yield of codeinone. In later work King et al. oxidized codeine with silver carbonate in refluxing toluene or xylene and obtained an 85% yield of codeinone with a much shorter reaction time.
General Description
Odorless yellow to brown solid. Sinks in water.
Reactivity Profile
Silver carbonate is a carbonates salt that has weak oxidizing or reducing powers. It is decomposed by acids with the evolution of carbon dioxide.
Health Hazard
Contact with eyes causes irritation. If continued for a long period, ingestion or inhalation of silver compounds can cause permanent discoloration of the skin (argyria).
Fire Hazard
Behavior in Fire: Decomposes to silver oxide, silver, and carbon dioxide; the reaction is not hazardous.
Properties of Silver carbonate
| Melting point: | 210 °C (dec.) (lit.) |
| Density | 6.08 g/mL at 25 °C (lit.) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | Aqueous Acid (Slightly), Aqueous Base (Slightly) |
| form | Granular Powder |
| color | Green-yellow to greenish |
| Specific Gravity | 6.08 |
| Water Solubility | insoluble |
| Sensitive | Light Sensitive |
| Merck | 14,8507 |
| Solubility Product Constant (Ksp) | pKsp: 11.07 |
| BRN | 6936654 |
| Stability: | Stability Stable, but light sensitive. Incompatible with reducing agents, acids. |
| CAS DataBase Reference | 534-16-7(CAS DataBase Reference) |
| NIST Chemistry Reference | silver carbonate(534-16-7) |
| EPA Substance Registry System | Silver(I) carbonate (534-16-7) |
Safety information for Silver carbonate
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Environment GHS09 |
| GHS Hazard Statements |
H318:Serious eye damage/eye irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P391:Collect spillage. Hazardous to the aquatic environment P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P501:Dispose of contents/container to..… |
Computed Descriptors for Silver carbonate
| InChIKey | KQTXIZHBFFWWFW-UHFFFAOYSA-L |
Silver carbonate manufacturer
Micron Platers
S D Fine Chem Limited
ARRAKIS INDUSTRIES LLP
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Silver carbonate 99%View Details
Silver carbonate 99%View Details -
 Silver carbonate on Celite, ∼0.7 mmole Ag<sub>2</sub>CO<sub>3</sub>/g reagent CAS 534-16-7View Details
Silver carbonate on Celite, ∼0.7 mmole Ag<sub>2</sub>CO<sub>3</sub>/g reagent CAS 534-16-7View Details
534-16-7 -
 Silver carbonate on Celite, ∼0.7 mmole Ag<sub>2</sub>CO<sub>3</sub>/g reagent CAS 534-16-7View Details
Silver carbonate on Celite, ∼0.7 mmole Ag<sub>2</sub>CO<sub>3</sub>/g reagent CAS 534-16-7View Details
534-16-7 -
 Silver carbonate CAS 534-16-7View Details
Silver carbonate CAS 534-16-7View Details
534-16-7 -
 Silver carbonate CAS 534-16-7View Details
Silver carbonate CAS 534-16-7View Details
534-16-7 -
 Silver Carbonate pure CAS 534-16-7View Details
Silver Carbonate pure CAS 534-16-7View Details
534-16-7 -
 Silver Carbonate CAS 534-16-7View Details
Silver Carbonate CAS 534-16-7View Details
534-16-7 -
 Silver carbonate 99% CAS 534-16-7View Details
Silver carbonate 99% CAS 534-16-7View Details
534-16-7