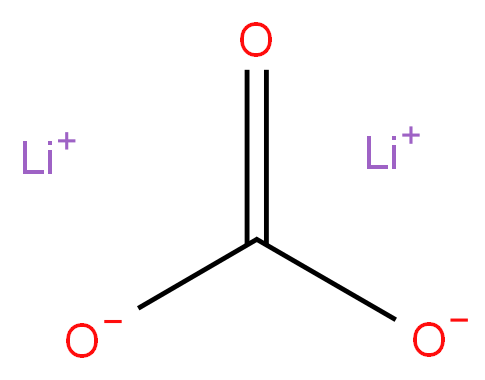
Lithium carbonate
Synonym(s):Carbolithium;Carbonic acid lithium salt;Lithium carbonate
- CAS NO.:554-13-2
- Empirical Formula: CLi2O3
- Molecular Weight: 73.89
- MDL number: MFCD00011084
- EINECS: 209-062-5
- Update Date: 2025-12-17 09:49:53
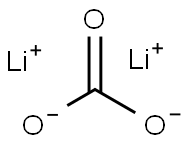
What is Lithium carbonate?
Absorption
Lithium absorption is rapid and oral bioavailability is close to 100%.
Toxicity
In rats, the oral LD50 is 525mg/kg and the inhalation LC50 is >2.17mg/L over 4 hours.
There is insufficient data regarding the carcinogenicity, mutagenicity, or fertility impairment of lithium carbonate. However, studies in rats and mice have shown repeated daily dosing of lithium carbonate result in adverse effects on male reproductive organs, spermatogenesis, and testosterone levels.
There is conflicting evidence regarding the incidence of cardiovascular abnormalities in first trimester administration of lithium. Animal studies have shown adverse effects on the fetus and fertility overall. The risk and benefit of lithium use in pregnancy must be weighed and should lithium treatment continue in pregnancy, serum lithium concentrations should be regularly monitored, dosages should be adjusted, and lithium should be decreased or stopped 2 or 3 days before delivery to avoid maternal and/or neonatal toxicity.
Breastfeeding is not recommended with maternal lithium use but if it is continued, the infant should be monitored for thyroid function and symptoms of lithium toxicity such as hypertonia, hypothermia, cyanosis, and ECG changes.
Description
Lithium carbonate is a white hygroscopicpowder. Molecular weight = 73.89; Boiling point = 1310℃(decomposes below BP); Freezing/Meltingpoint = 618-735℃. Hazard Identification (based onNFPA-704 M Rating System): Health 1, Flammability 0,Reactivity 1. Slightly soluble in water.
Background
Lithium has been used to treat manic episodes since the 19th century. Though it is widely used, its mechanism of action is still unknown. Lithium carbonate has a narrow therapeutic range and so careful monitoring is required to avoid adverse effects.
Indications
Lithium carbonate is indicated as a monotherapy for the treatment of acute manic and mixed episodes associated with bipolar 1 disorder in patients ≥7 years of age. It is also indicated as a maintenance treatment for bipolar 1 disorder in patients ≥7 years of age.
Pharmacokinetics
Lithium's mechanism of action is still unknown. Lithium's therapeutic action may be due to a number of effects, ranging from inhibition of enzymes such as glycogen synthase kinase 3, inositol phosphatases, or modulation of glutamate receptors.
Side Effects
Drowsiness, dizziness, tiredness, increased thirst, increased frequency of urination, weight gain, and mildly shaking hands (fine tremor) may occur. These should go away as your body adjusts to the medication. This medication may increase serotonin and rarely cause a very serious condition called serotonin syndrome/toxicity. The risk increases if you are also taking other drugs that increase serotonin, so tell your doctor or pharmacist of all the drugs you take (see Drug Interactions section). Get medical help right away if you develop some of the following symptoms: fast heartbeat, hallucinations, loss of coordination, severe dizziness, severe nausea/vomiting/diarrhea, twitching muscles, unexplained fever, unusual agitation/restlessness.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Medical observation is recommended for 24-48 h afterbreathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering a corticosteroid spray.
Metabolism
Lithium carbonate is not metabolized before excretion.
Properties of Lithium carbonate
| Melting point: | 720 °C |
| Boiling point: | 1342 °C(lit.) |
| Density | 2.11 g/mL at 25 °C |
| Flash point: | 1310°C |
| storage temp. | Store at +5°C to +30°C. |
| solubility | 13g/l |
| form | wire |
| pka | pKa 6.38 (Uncertain);10.25 (Uncertain) |
| color | White |
| Specific Gravity | 2.11 |
| PH | 10-11 (5g/l, H2O, 20℃) |
| Odor | odorless |
| Water Solubility | 13 g/L (20 ºC) |
| Merck | 14,5527 |
| Solubility Product Constant (Ksp) | pKsp: 1.6 |
| BRN | 3999191 |
| CAS DataBase Reference | 554-13-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Lithium carbonate(554-13-2) |
| EPA Substance Registry System | Lithium carbonate (554-13-2) |
Safety information for Lithium carbonate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Lithium carbonate
| InChIKey | XGZVUEUWXADBQD-UHFFFAOYSA-L |
Lithium carbonate manufacturer
JSK Chemicals
Sainor Laboratories Pvt Ltd Unit III
Vasa Pharmachem Pvt Ltd. (VPPL)
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 LITHIUM CARBONATE 99%View Details
LITHIUM CARBONATE 99%View Details -
 Lithium Carbonate 98%View Details
Lithium Carbonate 98%View Details -
 Lithium carbonate 99%View Details
Lithium carbonate 99%View Details -
 LITHIUM CARBONATE 99%View Details
LITHIUM CARBONATE 99%View Details -
 Lithium carbonate 98%View Details
Lithium carbonate 98%View Details -
 Lithium Carbonate CASView Details
Lithium Carbonate CASView Details -
 Lithium standard solution CASView Details
Lithium standard solution CASView Details -
 Lithium ICP Standard CASView Details
Lithium ICP Standard CASView Details
