Cesium carbonate
Synonym(s):Carbonic acid dicesium
- CAS NO.:534-17-8
- Empirical Formula: CCs2O3
- Molecular Weight: 325.82
- MDL number: MFCD00010957
- EINECS: 208-591-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-20 20:09:13
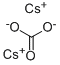
What is Cesium carbonate ?
Description
Cesium carbonate is a basic carbonate and is used in organic synthesis as a mild inorganic base. It can be used in C, N, O alkylation and arylation reactions. Cesium carbonate not only promotes the carbonylation of alcohols and carbamination of amines but also inhibits side reactions common in other processes. It is also used in six-membered annulation, intramolecular and intermolecular cyclizations, Suzuki coupling, aza-Henry, nucleophilic substitution, cross-coupling and different cycloaddition reactions.
Chemical properties
Colourless crystals or white powder , easily soluble in water, quickly absorbs moisture when placed in the air. The aqueous solution of cesium carbonate is strongly alkaline and can react with acid to produce the corresponding cesium salt and water, and release carbon dioxide. One of the biggest advantages of cesium carbonate (Cs2CO3) is that it has a higher solubility in most solvents than K2CO3 or Na2CO3.
The Uses of Cesium carbonate
Cesium carbonate is a versatile reagent for organic synthesis. It has also found use as a catalyst for ethylene oxide polymerization, in coating for spatter-free welding of steel in CO2, as a functional interlayer in photovoltaic devices and in oxide cathodes. Cesium carbonate is also used in the beer-brewing industry to make the "head" of beer foamier. Cesium carbonate is useful in the N-alkylation (of sulfonamides, beta-lactams, indoles, heterocycles and several sensitive nitrogen compounds), carbamination of amines, carbonylation of alcohols and aerobic oxidation of alcohols into carbonyl compounds without polymeric by products.
Preparation
cesium carbonate can be prepared by thermal decomposition of caesium oxalate. Upon heating, caesium oxalate is converted to caesium carbonate with emission of carbon monoxide.
Cs2C2O4 → Cs2CO3 + CO
It can also be synthesized by reacting caesium hydroxide with carbon dioxide.
2 CsOH + CO2 → Cs2CO3 + H2O
Safety Profile
Moderately toxic by ingestion. Mutation data reported. When heated to decomposition it emits acrid smoke and fumes. See also CESIUM.
References
[1] Guo L, et al. Applications of Bases in Transition Metal Catalyzed Reactions Zhao Yuan. Chemistry - A European Journal, 2018; 24: 7794-7809.
Properties of Cesium carbonate
| Melting point: | 610 °C (dec.) (lit.) |
| Density | 4.072 |
| vapor pressure | 0Pa at 25℃ |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | soluble in Water |
| form | Powder/Granules |
| appearance | White powder |
| Specific Gravity | 4.072 |
| color | White |
| Water Solubility | 261 g/100 mL (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,2010 |
| BRN | 4546405 |
| Stability: | Stable. Very deliquescent. Incompatible with strong oxidizing agents, strong acids. |
| CAS DataBase Reference | 534-17-8(CAS DataBase Reference) |
| EPA Substance Registry System | Carbonic acid, dicesium salt (534-17-8) |
Safety information for Cesium carbonate
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Health Hazard GHS08 |
| GHS Hazard Statements |
H318:Serious eye damage/eye irritation H373:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Cesium carbonate
| InChIKey | FJDQFPXHSGXQBY-UHFFFAOYSA-L |
Cesium carbonate manufacturer
Jaiswal S Cyber Shop
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Cesium carbonate CAS 534-17-8View Details
Cesium carbonate CAS 534-17-8View Details
534-17-8 -
 Cesium carbonate, (metals basis) CAS 534-17-8View Details
Cesium carbonate, (metals basis) CAS 534-17-8View Details
534-17-8 -
 Cesium carbonate CAS 534-17-8View Details
Cesium carbonate CAS 534-17-8View Details
534-17-8 -
 Cesium carbonate CAS 534-17-8View Details
Cesium carbonate CAS 534-17-8View Details
534-17-8 -
 Cesium CAS 534-17-8View Details
Cesium CAS 534-17-8View Details
534-17-8 -
 Cesium CAS 534-17-8View Details
Cesium CAS 534-17-8View Details
534-17-8 -
 Cesium carbonate CAS 534-17-8View Details
Cesium carbonate CAS 534-17-8View Details
534-17-8 -
 Cesium carbonate CAS 534-17-8View Details
Cesium carbonate CAS 534-17-8View Details
534-17-8
