Ammonium bicarbonate
Synonym(s):Ammonium bicarbonate, Ammonium carbonate;Ammonium hydrogen carbonate
- CAS NO.:1066-33-7
- Empirical Formula: CH5NO3
- Molecular Weight: 79.06
- MDL number: MFCD00012138
- EINECS: 213-911-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:40
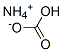
What is Ammonium bicarbonate?
Chemical properties
Ammonium bicarbonate is a white crystalline solid with a faint ammonia odor and soluble in water but insoluble in alcohol and acetone. It decomposes above 35℃ to ammonia, carbon dioxide and water vapor, releasing irritant fumes. Only 30% of the applied nitrogen of this fertilizer is recovered by plants owing to the unstable nature of ammonium bicarbonate. that forms by the reaction of anunonium hydroxide and excess CO2.
Physical properties
White crystalline solid; prismatic crystal; faint odor of ammonia; stable at ambient temperature but decomposes on heating at 60°C; melts at 107.5°C on very rapid heating; density 1.586 g/cm3; vapor pressure 435 torr at 25°C; readily dissolves in water (21.6g/100g at 20°C, and 36.6g/100g at 40°C).
The Uses of Ammonium bicarbonate
Ammonium Bicarbonate is a dough strengthener, a leavening agent, a ph control agent, and a texturizer. prepared by reacting gaseous carbon dioxide with aqueous ammonia. crystals of ammo- nium bicarbonate are precipitated from solution and subsequently washed and dried. Also known as hartshorn and rock ammonia, ammonium bicarbonate is soluble in water but decomposes when heated. It was used in place of ammonia when making ammonia-ripened gelatin emulsions.
The Uses of Ammonium bicarbonate
Ammonium bicarbonate is a commonly used reagent for industrial and research procedures. It acts a good buffer in lyophilization and matrix assisted laser desorption. It is also utilized for the in-gel digestion of proteins by trypsin and in the MALDI mass spectrometric analysis of proteins. It is also used to make other ammonium compounds, in food processing, and for other uses. Ammonium bicarbonate can be used to study biological buffers. It is also used in a study that demonstrated that ammonium bicarbonate salts, which can be regenerated using low-temperature waste heat, can also produce sufficient voltage for hydrogen gas generation in a microbial reverse-electrodialysis electrolysis cells. It has also been used in a study that developed a fast and sensitive method for the simultaneous determination of Sudan dyes in food samples using partial filling micellar electrokinectic chromatography-mass spectrometry.
What are the applications of Application
Ammonium bicarbonate is a buffer applications such as lyophilization and matrix assisted laser desorption
Definition
ChEBI: Ammonium bicarbonate is an organooxygen compound. It is a buffer applications such as lyophilization and matrix assisted laser desorption.
General Description
A white crystalline solid having the odor of ammonia. Soluble in water. The primary hazard is the threat to the environment. Immediate steps should be taken to limit spread to the environment. Used to make other ammonium compounds, in food processing, and for other uses.
Air & Water Reactions
Soluble in water.
Reactivity Profile
Heat > 36°C ( produces ammonia and carbon dioxide); strong acids and strong bases (CO2 and NH3) [Handling Chemicals Safely 1980 p. 141].
Hazard
Evolves irritating fumes on heating to 35C.
Health Hazard
Inhalation may cause respiratory irritation. Ingestion could be harmful. Contact with eyes or skin causes irritation.
Breathing Ammonium Bicarbonate can irritate the nose, throat, and lungs causing coughing, wheezing, and/or shortness of breath. No risks to humans are expected from approved uses of ammonium bicarbonate as a pesticide active ingredient. The substance is approved as a food additive by the Food and Drug Administration (FDA) and as an inert pesticide ingredient by EPA. No toxic endpoints have been identified.
Agricultural Uses
Ammonium hydrogen carbonate is another name for ammonium bicarbonate (NH4CO3), It is a low nitrogen containing fertilizer (17% N), used largely in China. It is produced by heating ammonium hydroxide with excess carbon dioxide, followed by evaporation of water.
Safety Profile
Poison by intravenous route. When heated to decomposition it emits toxic fumes of NO, and NH3
Potential Exposure
It is used in leavening for some baked goods; in baking powders and fire extinguishers; to make dyes and pigments; in the manufacture of porous plastics; and as an expectorant.
Shipping
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Incompatibilities
Contact with strong caustics, such as potassium hydroxide or sodium hydroxide will cause the release of ammonia gas. Decomposes as temperature rises >35 C.
Waste Disposal
May be buried in a chemical waste landfill. If neutralized ammonium bicarbonate is amenable to treatment at a municipal sewage treatment plant.
Properties of Ammonium bicarbonate
| Melting point: | 105 °C |
| Boiling point: | 143.04°C (rough estimate) |
| Density | 1,586 g/cm3 |
| vapor density | 2.7 (vs air) |
| vapor pressure | 67 hPa (20 °C) |
| refractive index | 1.4164 (estimate) |
| storage temp. | 2-8°C |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | Solid |
| color | White |
| Odor | faint ammonia odor |
| PH | 7.0-8.5 (25℃, 1M in H2O) |
| Water Solubility | 220 g/L (20 ºC) |
| λmax | λ: 260 nm Amax: ≤0.030 λ: 280 nm Amax: ≤0.020 |
| Merck | 14,497 |
| BRN | 4329606 |
| Stability: | Stable. Incompatible with strong acids, alkali metals. |
| CAS DataBase Reference | 1066-33-7(CAS DataBase Reference) |
| EPA Substance Registry System | Ammonium bicarbonate (1066-33-7) |
Safety information for Ammonium bicarbonate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for Ammonium bicarbonate
Ammonium bicarbonate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Ammonium Bi Carbonate 99%View Details
Ammonium Bi Carbonate 99%View Details -
 AMMONIUM BICARBONATE 98%View Details
AMMONIUM BICARBONATE 98%View Details -
 Ammonium bicarbonate 99%View Details
Ammonium bicarbonate 99%View Details -
 Ammonium bicarbonate 98%View Details
Ammonium bicarbonate 98%View Details -
 Ammonium hydrogen carbonate CAS 1066-33-7View Details
Ammonium hydrogen carbonate CAS 1066-33-7View Details
1066-33-7 -
 Ammonium hydrogen carbonate CAS 1066-33-7View Details
Ammonium hydrogen carbonate CAS 1066-33-7View Details
1066-33-7 -
 Ammonium Bicarbonate ChemicalView Details
Ammonium Bicarbonate ChemicalView Details
1066-33-7 -
 Ammonium Bicarbonate Food Grade, 99.5%, 25kg BagView Details
Ammonium Bicarbonate Food Grade, 99.5%, 25kg BagView Details
1066-33-7
