Salicylhydroxamic acid
Synonym(s):N,2-Dihydroxybenzamide;2-Hydroxybenzohydroxamic acid
- CAS NO.:89-73-6
- Empirical Formula: C7H7NO3
- Molecular Weight: 153.14
- MDL number: MFCD00002110
- EINECS: 201-934-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:52
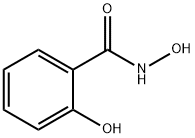
What is Salicylhydroxamic acid?
Chemical properties
It is a white needle crystal or off-white powder with a slow-heating melting point of 168°C. It is soluble in alcohol and ether and can be sublimated. When exposed to air, it progressively turns red. It is synthesized by combining methyl salicylate and hydroxylamine hydrochloride with sodium hydroxide, followed by acidification.
The Uses of Salicylhydroxamic acid
Salicylhydroxamic acid is a collector with efficient chelating effect on rare metal oxide minerals. It is used in rare metal beneficiation and has the advantages of good selectivity and strong collecting power. Salicylhydroxamic acid is mainly used as a complexing agent or extractant for rare earth ore, copper oxide ore, lead-zinc oxide ore, gold ore, kaolin, etc., as well as organic synthesis intermediates.
What are the applications of Application
Salicylhydroxamic acid is an irreversible inhibitor of bacterial and plant urease
Definition
ChEBI: Salicylhydroxamic acid is a hydroxamic acid that is N-hydroxybenzamide carrying a phenolic hydroxy group at position 2. It has a role as an antibacterial drug, a trypanocidal drug, an EC 3.5.1.5 (urease) inhibitor and an EC 1.11.2.2 (myeloperoxidase) inhibitor. It is a member of phenols and a hydroxamic acid.
What are the applications of Application
Salicylhydroxamic acid has various applications, such as:
Synthesizing phenylboronic acid-based bioconjugates for chromatographic use.
Acting as a ligand to create complexes of Fe(III), Cu(II), Ni(II), and Zn(II).
Acting as a selective collector during the flotation technique for segregating oxide minerals.
Preparation
The synthesis of salicylhydroxamic acid involved a two-step process. In the first step, methyl salicylate was prepared by esterifying salicylic acid with methanol using 28g (0.2 mol) of salicylic acid, 81 ml of methanol, and 8 ml of concentrated sulfuric acid. In the second step, hydroxylamine was coupled with the methyl salicylate. 13.9 g (0.2mol) of hydroxylamine was mixed with 200 ml of 10% sodium hydroxide solution and cooled. To this, 152.3g (0.1mol) of methyl salicylate was added in small portions with vigorous shaking after each addition to ensure complete dissolution. The mixture was allowed to stand for 36 hours, then acidified with 2M sulphuric acid and cooled. The precipitate was filtered, recrystallized from hot water containing a drop of acetic acid, recooled, and filtered. The yield of white precipitate collected and weighed was 14g of salicylhydroxamic acid.
Preparation and Characterization of Salicylhydroxamic acid and its Complexes with Iron (III), Cobalt (II) and Manganese (II)
Purification Methods
Crystallise the hydroxamic acid from acetic acid. [Beilstein 10 H 98.]
Properties of Salicylhydroxamic acid
| Melting point: | 177 °C (dec.) (lit.) |
| Boiling point: | 276.03°C (rough estimate) |
| Density | 1.3585 (rough estimate) |
| refractive index | 1.5500 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| form | Fine Crystalline Powder |
| pka | pK (25°) 4.19 |
| color | Beige-pink |
| Water Solubility | It is soluble in water. |
| Merck | 14,8331 |
| BRN | 1210520 |
| Stability: | Stable. Incompatible with strong bases, strong oxidizing agents. Combustible. |
| CAS DataBase Reference | 89-73-6(CAS DataBase Reference) |
| EPA Substance Registry System | N,2-Dihydroxybenzamide (89-73-6) |
Safety information for Salicylhydroxamic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H351:Carcinogenicity |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Salicylhydroxamic acid
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran






You may like
-
 89-73-6 Salicylhydroxamic acid 98%View Details
89-73-6 Salicylhydroxamic acid 98%View Details
89-73-6 -
 89-73-6 Salicylhydroxamic acid 98%View Details
89-73-6 Salicylhydroxamic acid 98%View Details
89-73-6 -
 Salicylhydroxamic Acid CAS 89-73-6View Details
Salicylhydroxamic Acid CAS 89-73-6View Details
89-73-6 -
 Salicylhydroxamic acid 97% CAS 89-73-6View Details
Salicylhydroxamic acid 97% CAS 89-73-6View Details
89-73-6 -
 Salicylhydroxamic acid 99% (HPLC) CAS 89-73-6View Details
Salicylhydroxamic acid 99% (HPLC) CAS 89-73-6View Details
89-73-6 -
 Salicylhydroxamic acid CAS 89-73-6View Details
Salicylhydroxamic acid CAS 89-73-6View Details
89-73-6 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
