Benzohydroxamic acid
Synonym(s):N-Hydroxybenzamide
- CAS NO.:495-18-1
- Empirical Formula: C7H7NO2
- Molecular Weight: 137.14
- MDL number: MFCD00002109
- EINECS: 207-797-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
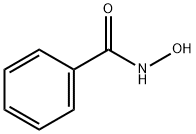
What is Benzohydroxamic acid?
Chemical properties
Crystalline Solid
The Uses of Benzohydroxamic acid
Benzhydroxamic acid may be employed for the Pd-catalyzed synthesis of benzisoxazolones.
The Uses of Benzohydroxamic acid
Benzhydroxamic acid is used as precursor in the synthesis of novel mono-anionic and di-anionic hydroxamato complexes by reacting with BiPh3 and Bi(O(t)Bu)3, which has anti-bacterial activity against helicobacter pylori. It is used in the photometric determination of trace amounts of vanadium in alloy steels by making mixed-ligand vanadium chelates with ammonium thiocyanate. It is also involved in the palladium catalyzed synthesis of benzisoxazolones.
Preparation
To a rapidly stirred suspension of 19.6 gm (0.35 mole) of powdered anhydrous potassium hydroxide in 120 ml of pyridine, maintained at 0-5°C, is a added a solution of 13.9 gm (0.2 mole) of hydroxylamine hydrochloride in 100 ml of pyridine.
While maintaining the reaction temperature at 0-5°C, 15 gm (0.1 mole) of ethyl benzoate is added. Vigorous stirring is continued at room temperature for 6 hr. Then the solids are filtered off. The solids are washed with cold water to remove inorganic coproducts. The remainder, recrystallized from aqueous ethanol, represents a 94% yield of potassium benzohydroxamate.
This salt is triturated with cold 0.01 TV hydrochloric acid. From this mixture, by the usual procedures, 12.5 gm (91% overall) of benzohydroxamic acid is isolated, m.p. 131°C (from aqueous alcohol).
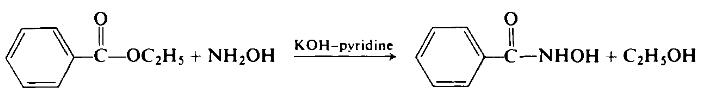
Synthesis Reference(s)
Tetrahedron Letters, 33, p. 5055, 1992 DOI: 10.1016/S0040-4039(00)61187-5
General Description
Rhombic crystals or light beige solid.
Air & Water Reactions
Slightly soluble in water.
Reactivity Profile
Benzohydroxamic acid is an amide. Amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx).
Health Hazard
ACUTE/CHRONIC HAZARDS: When heated to decomposition Benzohydroxamic acid emits toxic fumes of nitrogen oxides.
Fire Hazard
Flash point data for Benzohydroxamic acid are not available; however, Benzohydroxamic acid is probably combustible.
Safety Profile
Moderately toxic by ingestion.Mutation data reported. When heated to decomposition itemits toxic fumes of NOx.
Properties of Benzohydroxamic acid
| Melting point: | 126-130 °C(lit.) |
| Boiling point: | 251.96°C (rough estimate) |
| Density | 1.2528 (rough estimate) |
| refractive index | 1.5030 (estimate) |
| storage temp. | 2-8°C |
| solubility | Soluble in alcohol. Slightly soluble in ether. Insoluble in benzene. |
| form | Solid |
| pka | pK1: 8.89(0) (20°C) |
| color | Rhombic tablets |
| Water Solubility | 22 g/L (6 ºC) |
| BRN | 1907585 |
| Stability: | Moisture and Temperature Sensitive |
| CAS DataBase Reference | 495-18-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzamide, n-hydroxy-(495-18-1) |
| EPA Substance Registry System | Benzohydroxamic acid (495-18-1) |
Safety information for Benzohydroxamic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H341:Germ cell mutagenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Benzohydroxamic acid
| InChIKey | VDEUYMSGMPQMIK-UHFFFAOYSA-N |
Benzohydroxamic acid manufacturer
Dr. Silviu Pharmachem Pvt., Ltd.
Anand Agencies
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran





You may like
-
 495-18-1 BENZHYDROXAMIC ACID(BHA) 99%View Details
495-18-1 BENZHYDROXAMIC ACID(BHA) 99%View Details
495-18-1 -
 495-18-1 98%View Details
495-18-1 98%View Details
495-18-1 -
 Benzohydroxamic acid 98%View Details
Benzohydroxamic acid 98%View Details
495-18-1 -
 Benzohydroxamic Acid CAS 495-18-1View Details
Benzohydroxamic Acid CAS 495-18-1View Details
495-18-1 -
 Benzohydroxamic acid 98% CAS 495-18-1View Details
Benzohydroxamic acid 98% CAS 495-18-1View Details
495-18-1 -
 Benzhydroxamic acid CAS 495-18-1View Details
Benzhydroxamic acid CAS 495-18-1View Details
495-18-1 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1