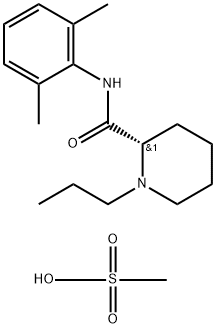
Ropivacaine
Synonym(s):(2S)-N-(2,6-Dimethylphenyl)-1-propyl-2-piperidinecarboxamide;Ropivacaine
- CAS NO.:84057-95-4
- Empirical Formula: C17H26N2O
- Molecular Weight: 274.4
- MDL number: MFCD00864425
- EINECS: 200-001-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
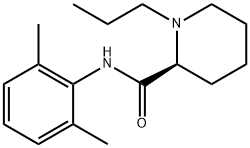
What is Ropivacaine?
Absorption
Ropivacaine pharmacokinetics are highly dependent on the dose, route of administration, and patient condition. Following epidural administration ropivacaine undergoes complete and biphasic absorption.
Toxicity
High systemic doses of ropivacaine can result in central nervous system (CNS) and cardiovascular effects, with the CNS effects usually occurring at lower blood plasma concentrations and additional cardiovascular effects occurring at higher concentrations (although cardiovascular collapse may occur at lower concentrations). CNS effects include CNS excitation involving nervousness, tingling around the mouth, tinnitus, tremor, dizziness, blurred vision, and seizures. CNS depressant effects may follow, associated with drowsiness, loss of consciousness, respiratory depression and apnea. Cardiovascular events may be caused by hypoxemia secondary to respiratory depression and include hypotension, bradycardia, arrhythmias, and/or cardiac arrest.
Description
Ropivacaine is an aminoamide local anaesthetic drug commonly marketed by AstraZeneca under the trade name Naropin. Naropin was launched in 1996 in Australia, Denmark, Finland, the Netherlands and Sweden as a local anesthetic. It can be prepared in a number of ways the most efficient involves a three step sequence beginning with L-pipecolic acid. This compound is the first one in this family to be produced as the pure (S)- enantiomer. The (R)-enantiomer has been shown to have cardiotoxic effects. Thus ropivicaine has less cardiovascular and CNS toxicity than bupivacaine. It is a Na channel blocker that is specific for affecting nerve fibers responsible for transmission of pain (Aδ and C) with no effect on fibers responsible for motor function (Aβ). Clinically, it has distinct advantages over bupivacaine. Its effects are slower in onset, less intense and have a shorter duration. This is a result of extensive metabolism in the liver to the 3-hydroxy isomer by CYPIA2 isoenzyme.
Ropivacaine is an anesthetic (numbing medicine) that blocks the nerve impulses that send pain signals to your brain.
Ropivacaine is used as a local (in only one area) anesthesia for a spinal block, also called an epidural. The medication is used to provide anesthesia during a surgery or C-section, or to ease labor pains.
https://www.drugs.com/mtm/ropivacaine.html
Originator
Astra (Sweden)
The Uses of Ropivacaine
Ropivacaine is a pure S(-)-enantiomer of propivacaine. It is a long-acting amide local anesthetic that has efficacy and potency nearly as high as bupivacaine and levobupivacaine but has lower CNS and cvS toxicity. If there are any differences between bupivacaine and ropivacaine it is the slightly shorter period of activity in spinal and epidural applications and a lowered ability to penetrate large motor nerves. It is the reduced lipophilicity that contributed to the lowered penetration of the motor nerves, combined with its stereoselective properties that allows ropivacaine to have significantly reduced Cvs toxicity compared to bupivacaine (Simpson et al. 2005). Ropivacaine has a diphasic effect on peripheral vasculature-it is vasoconstrictive when injected at a concentration below 0.5 w/v% and there is dilation at concentrations over 1 w/v% (Cederholm et al. 1992).
Ropivacaine HCl is an anaesthetic agent and blocks impulse conduction in nerve fibres through inhibiting sodium ion influx reversibly.
http://www.selleckchem.com/products/ropivacaine-hcl.html
The Uses of Ropivacaine
(S)-Ropivacaine acts as a local anaesthetic for use during medical procedures. Frequently used in epidural procedures as a labor analgesia
Indications
Ropivacaine is indicated in adult patients for the induction of regional or local anesthesia for surgery or acute pain management.
Background
Ropivacaine is an aminoamide local anesthetic drug marketed by AstraZeneca under the trade name Naropin. It exists as a racemate of its S- and R-enantiomers, although the marketed form is supplied only as the purified S-enantiomer.
Definition
ChEBI: A piperidinecarboxamide-based amide-type local anaesthetic (amide caine) in which (S)-N-propylpipecolic acid and 2,6-dimethylaniline are combined to form the amide bond.
brand name
Narapin [as hydrochloride] (Astra).
Biological Functions
Ropivacaine (Naropin) is a recently developed longacting amide-linked local anesthetic. Its duration of action is similar to that of bupivacaine, but it is slightly less potent and requires higher concentrations to achieve the same degree of block. Its primary advantage over bupivacaine is its lesser degree of cardiotoxicity.
General Description
The recognized increase in cardiotoxicity of one bupivacaineisomer led to the stereospecific production of ropivacaine asthe single “S” (-) enantiomer. Ropivacaine is the propylanalog of mepivacaine (methyl) and bupivacaine (butyl). ThepKa of the tertiary nitrogen is 8.1, and it displays the same degreeof protein binding as bupivacaine ( 94%). Althoughropivacaine has similar properties as bupivacaine, it displaysless cardiotoxicity. The shortened alkyl chain gives it approximatelyone third of the lipid solubility of bupivacaine.
Mechanism of action
Ropivacaine is a member of the amino amide class of local anesthetics and is supplied as the pure S-(- )-enantiomer. Local anesthetics block the generation and the conduction of nerve impulses, presumably by increasing the threshold for electrical excitation in the nerve, by slowing the propagation of the nerve impulse, and by reducing the rate of rise of the action potential. In general, the progression of anesthesia is related to the diameter, myelination and conduction velocity of affected nerve fibers. Clinically, the order of loss of nerve function is as follows: (1) pain, (2) temperature, (3) touch, (4) proprioception, and (5) skeletal muscle tone.
Pharmacokinetics
In contrast to most other local anesthetics, the presence of epinephrine does not affect the time of onset, duration of action, or the systemic absorption of ropivacaine.
Pharmacology
This is a single (S–) enantiomer, similar in structure to bupivacaine. S ubstitution of a propyl for the butyl side chain of bupivacaine reduces lipid solubility; this leads to reduced potential for toxicity and also greater separation between sensory and motor blockade. Efficacy is similar, but motor block is reduced compared with equianalgesic doses of racemic bupivacaine.
Clinical Use
Ropivacaine is a long-acting amide-type local anestheticwith inherent vasoconstrictor activities, so it does not requirethe use of additional vasoconstrictors. It is approved forepidural, nerve block, infiltration, and intrathecal anesthesia.
Side Effects
Reactions to ropivacaine are characteristic of those associated with other amide-type local anesthetics. A major cause of adverse reactions to this group of drugs may be associated with excessive plasma levels, which may be due to overdosage, unintentional intravascular injection or slow metabolic degradation.
Check with your doctor or nurse immediately if any of the following side effects occur:
More common
Blurred vision
chest pain or discomfort
confusion
dizziness, faintness, or lightheadedness when getting up suddenly from a lying or sitting position
lightheadedness, dizziness, or fainting
slow or irregular heartbeat
sweating
unusual tiredness or weakness
Less common
Burning, crawling, itching, numbness, prickling, "pins and needles", or tingling feelings
chills
decrease in frequency or amount of urine
difficulty in passing urine (dribbling)
fever
painful urination
Rare
Absence of or decrease in body movement
agitation
anxiety
https://www.mayoclinic.org/drugs-supplements/ropivacaine-injection-route/side-effects/drg-20065875
https://www.accessdata.fda.gov/
Metabolism
Ropivacaine undergoes extensive metabolism, primarily via CYP1A2-mediated aromatic hydroxylation to 3-OH-ropivacaine. The main metabolites excreted in the urine are the N-dealkylated metabolite (PPX) and 3-OH-ropivacaine. Other identified metabolites include 4-OH-ropivacaine, the 3-hydroxy-N-dealkylated (3-OH-PPX) and 4-hydroxy-N-dealkylated (4-OH-PPX) metabolites, and 2-hydroxy-methyl-ropivacaine (which has been identified but not quantified).
Unbound PPX, 3-hydroxy-, and 4-hydroxy-ropivacaine have demonstrated pharmacological activity in animal models less than that of ropivacaine.
Metabolism
The metabolism of ropivacaine in human is mediated by hepatic CYP1A2 isozymes and, to a minor extent, by CYP3A4. The major metabolite is 3-hydroxyropivacaine, and the minor metabolite is (S)-2′,6′-pipecoloxylidide (a N-dealkylated product).
Ropivacaine is extensively metabolized in the liver, predominantly by aromatic hydroxylation mediated by cytochrome P4501A to 3-hydroxyropivacaine. After a single IV dose approximately 37% of the total dose is excreted in the urine as both free and conjugated 3-hydroxy ropivacaine. Low concentrations of 3-hydroxy ropivacaine have been found in the plasma. Urinary excretion of the 4-hydroxy ropivacaine, and both the 3-hydroxy N-de-alkylated (3-OH-PPX) and 4-hydroxy N-dealkylated (4-OH-PPX) metabolites account for less than 3% of the dose. An additional metabolite, 2- hydroxy-methyl-ropivacaine, has been identified but not quantified in the urine. The N-de-alkylated metabolite of ropivacaine (PPX) and 3-OH-ropivacaine are the major metabolites excreted in the urine during epidural infusion. Total PPX concentration in the plasma was about half as that of total ropivacaine; however, mean unbound concentrations of PPX were about 7 to 9 times higher than that of unbound ropivacaine following continuous epidural infusion up to 72 hours. Unbound PPX, 3- hydroxy and 4-hydroxy ropivacaine, have a pharmacological activity in animal models less than that of ropivacaine. There is no evidence of in vivo racemization in urine of ropivacaine.
https://www.accessdata.fda.gov
Properties of Ropivacaine
| Melting point: | 144-146° |
| Boiling point: | 410.2±45.0 °C(Predicted) |
| alpha | D25 -82.0° (c = 2 in methanol) |
| Density | 1.044±0.06 g/cm3(Predicted) |
| storage temp. | Refrigerator |
| solubility | Aqueous Acid (Slightly), Chloroform (Slightly), DMSO (Slightly), Methanol (Sligh |
| form | Solid |
| pka | 8.16(at 25℃) |
| color | White to Off-White |
| CAS DataBase Reference | 84057-95-4(CAS DataBase Reference) |
Safety information for Ropivacaine
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H336:Specific target organ toxicity,single exposure; Narcotic effects H370:Specific target organ toxicity, single exposure |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. |
Computed Descriptors for Ropivacaine
| InChIKey | ZKMNUMMKYBVTFN-HNNXBMFYSA-N |
Ropivacaine manufacturer
Dishman Carbogen Amcis Ltd (Dishman Group)
Apothecon Pharmaceuticals Pvt Ltd
SMS Lifesciences India Ltd (MAHI Drugs Pvt Ltd)
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 84057-95-4 (2S)-N-(2,6-Dimethylphenyl)-1-propyl-2- piperidinecarboxamide 98%View Details
84057-95-4 (2S)-N-(2,6-Dimethylphenyl)-1-propyl-2- piperidinecarboxamide 98%View Details
84057-95-4 -
 84057-95-4 98%View Details
84057-95-4 98%View Details
84057-95-4 -
 Ropivacaine 98% (GC) CAS 84057-95-4View Details
Ropivacaine 98% (GC) CAS 84057-95-4View Details
84057-95-4 -
 Ropivacaine 99% (GC) CAS 84057-95-4View Details
Ropivacaine 99% (GC) CAS 84057-95-4View Details
84057-95-4 -
 84057-95-4 Ropivacaine 98%View Details
84057-95-4 Ropivacaine 98%View Details
84057-95-4 -
 Ropivacaine 99%View Details
Ropivacaine 99%View Details
84057-95-4 -
 Ropivacaine CAS 84057-95-4View Details
Ropivacaine CAS 84057-95-4View Details
84057-95-4 -
 Ropivacaine CAS 84057-95-4View Details
Ropivacaine CAS 84057-95-4View Details
84057-95-4