Propyl gallate
Synonym(s):3,4,5-Trihydroxybenzoic acid propyl ester;PG;Propyl 3,4,5-trihydroxybenzoate;Propyl gallate, Gallic acid propyl ester, 3,4,5-Trihydroxybenzoic acid propyl ester
- CAS NO.:121-79-9
- Empirical Formula: C10H12O5
- Molecular Weight: 212.2
- MDL number: MFCD00002196
- EINECS: 204-498-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-22 18:23:36
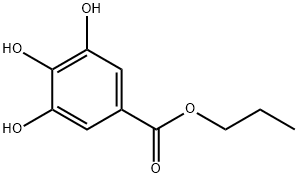
What is Propyl gallate?
Description
Propyl gallate is an antioxidant with antimicrobial activity. It is hepatoprotective in vitro and in vivo, preventing CCl4 induced lipoperoxidation and reduction in polysomes in rat liver. Propyl gallate (100 mg/kg, i.p.) increases expression of HIF-1α, EPO, and VEGF mRNA levels and the number of normal neurons in rat brains after 8 minutes of forebrain ischemia. Propyl gallate in combination with potassium sorbate is bactericidal and bacteriostatic against S. aureus strains known to produce enterotoxins in food. Propyl gallate is commonly added to foods to prevent autoxidation and microbial growth.
Description
n-Propyl gallate is an antioxidant for oils and fats that is used in foods, cosmetics, adhesives, and industrial lubricants. In 1983, M. A. Augustin and S. K. Berry were among the first researchers to study its antioxidant effectiveness. Recently, A. K. Franz and co-workers at the University of California, Davis, discovered a new property of?n-prolyl gallate:?It enhances the ability of microalgae to produce triacylglycerols. This finding may make it practical to use algae-derived lipids as biofuels.
Description
It caused contact dermatitis in a baker and in a female confectioner who fried doughnuts, primarily sensitized by her night cream; the margarine probably contained gallates.
Chemical properties
white to light beige crystalline powder
Chemical properties
Propyl gallate is an odorless powder having a slightly bitter taste. It functions particularly well in stabilizing animal fats and vegetable oils. With a melting point of 148°C, propyl gallate loses its effectiveness during heat processing and is therefore not suitable in frying applications that involve temperatures exceeding 190°C. Propyl gallate chelates iron ions and forms an unappealing, blue–black complex. Hence, it is always used with chelators such as citric acid to eliminate the pro-oxidative iron and copper catalysts. Good synergism is obtained with BHA and BHT; however, application with TBHQ is not permitted. For additional details, refer to Burdock (1997).
The Uses of Propyl gallate
propyl gallate is an anti-oxidant with preservative properties.
The Uses of Propyl gallate
Propyl Gallate is a known inhibitor of Tyrosinase, a polyphenol oxidase, which is an important enzyme in pigment biosynthesis in various organisms. It has also recently been seen to boost biodiesel li pid biosynthesis in cultures.
The Uses of Propyl gallate
Antioxidant for cosmetics, foods, fats, oils, ethers, emulsions, waxes, transformer oils.
The Uses of Propyl gallate
Propyl Gallate is an antioxidant that is the n-propylester of 3,4,5-tri- hydroxybenzoic acid. natural occurrence of propyl gallate has not been reported. it is commercially prepared by esterification of gallic acid with propyl alcohol followed by distillation to remove excess alcohol.
What are the applications of Application
Propyl gallate is a biochemical used as an anti-fade reagent in fluorescence microscopy
Preparation
Produced commercially by the esterification of gallic acid with propyl alcohol followed by distillation to remove the excess alcohol.
Definition
ChEBI: N-propyl gallate is a trihydroxybenzoic acid.
Production Methods
The preparation process of propyl gallate incldues the following steps: under the action of toluene-p-sulfonic acid as catalyst making gallic acid and n-propyl alcohol produce reaction at high temp., reflux and dehydration for 4-7 hr., distilling to recover excess solvent, dissolving residue by adding water, cooling, centrifuging (or filtering) to obtain crude product, decolouring and drying to obtain finished product. Its product propyl gallate yield rate can be up to 98%, and its cost is low.
General Description
Fine white to creamy-white crystalline powder. Odorless or with a faint odor. Melting point 150°C. Insoluble in water. Slightly bitter taste.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Propyl gallate can react with oxidizing agents. Incompatible with strong acids, strong bases and strong reducing agents. Darkens in the presence of iron and iron salts. Contact with metals should be avoided .
Hazard
Use in foods restricted to 0.02% of fat con- tent.
Fire Hazard
Propyl gallate is combustible.
Flammability and Explosibility
Not classified
Contact allergens
This gallate ester (E 311) is an antioxidant frequently used in the food, cosmetic, and pharmaceutical industries to prevent the oxidation of unsaturated fatty acids into rancid-smelling compounds. It causes cosmetic dermatitis mainly from lipsticks and induced contact dermatitis in a baker, and in a female confectioner, primarily sensitized by her night cream, who fried doughnuts the margarine probably containing gallates.
Biochem/physiol Actions
An antioxidant that exhibits antimicrobial activity. Propyl gallate has been reported to be an effective antioxidant-based hepatoprotector, both in vitro and in vivo. It has also been shown to prevent neuronal apoptosis and block the death of neurons exposed to FeSO4/GA as well as partially protect endothelial cells against TNF-induced apoptosis.
Safety Profile
Poison by ingestion and intraperitoneal routes. Experimental teratogenic and reproductive effects. Questionable carcinogen with experimental tumorigenic data. Mutation data reported. Combustible when exposed to heat or flame; can react with oxidizing materials. When heated to decomposition it emits acrid smoke and irritating fumes.
Toxicology
Propyl gallate (n-propyl-3,4,5-trihydroxybenzoate) is used in vegetable oils and butter. When 1.2 or 2.3% propyl gallate was added to feed for rats, loss of weight was observed. This may be due to the rats reluctance to eat food that was contaminated with the bitter taste of propyl gallate. When it was given for 10 to 16 months at the 2 to 3% level, 40% of the rats died within the first month and the remainder showed severe growth inhibition. Autopsies of rats indicated kidney damage resulting from the ingestion of propyl gallate. However, no other animal studies show serious problems and further studies indicated that propyl gallate does not cause serious chronic toxicities.
Safety
Propyl Gallate currently is used as an antioxidant in a reported 167 cosmetic products at maximum concentrations of 0.1%. Propyl Gallate is a generally recognized as safe (GRAS) antioxidant to protect fats, oils, and fat-containing food from rancidity that results from the formation of peroxides.
Purification Methods
Crystallise the ester from aqueous EtOH or *C6H6 (m 146-146.5o). [Beilstein 10 III 2078, 10 IV 2003.]
Properties of Propyl gallate
| Melting point: | 146-149 °C(lit.) |
| Boiling point: | 312.03°C (rough estimate) |
| Density | 1.210 |
| vapor pressure | 0Pa at 20℃ |
| refractive index | 1.5140 (estimate) |
| FEMA | 2947 | PROPYL GALLATE |
| Flash point: | 187℃ |
| storage temp. | Store below +30°C. |
| solubility | ethanol: 50 mg/mL |
| form | powder |
| pka | 8.11(at 25℃) |
| color | White to light beige |
| Odor | bland |
| Water Solubility | 0.35 g/100 mL (25 ºC) |
| Merck | 14,7859 |
| BRN | 1877976 |
| CAS DataBase Reference | 121-79-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzoic acid, 3,4,5-trihydroxy-, propyl ester(121-79-9) |
| EPA Substance Registry System | Propyl gallate (121-79-9) |
Safety information for Propyl gallate
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H317:Sensitisation, Skin H318:Serious eye damage/eye irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Propyl gallate
| InChIKey | ZTHYODDOHIVTJV-UHFFFAOYSA-N |
Propyl gallate manufacturer
JSK Chemicals
Pachisia Chemical Works
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Propyl gallate 99%View Details
Propyl gallate 99%View Details -
 Propyl gallate 99%View Details
Propyl gallate 99%View Details -
 Propyl gallate 98%View Details
Propyl gallate 98%View Details -
 Propyl Gallate 99%View Details
Propyl Gallate 99%View Details -
 Propyl Gallate IP CAS 121-79-9View Details
Propyl Gallate IP CAS 121-79-9View Details
121-79-9 -
 Propyl Gallate extrapure CAS 121-79-9View Details
Propyl Gallate extrapure CAS 121-79-9View Details
121-79-9 -
 Propyl Gallate, 1 kg PacketView Details
Propyl Gallate, 1 kg PacketView Details
121-79-9 -
 Propyl Gallate, For Feed & Feed premix, 25 kg BagView Details
Propyl Gallate, For Feed & Feed premix, 25 kg BagView Details
121-79-9
