Potassium sulfate
Synonym(s):Dipotassium Salt;Potassium pyrosulfate;Potassium sulfate
- CAS NO.:7778-80-5
- Empirical Formula: K2O4S
- Molecular Weight: 174.2592
- MDL number: MFCD00011388
- EINECS: 231-915-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:42
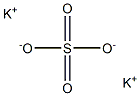
What is Potassium sulfate?
Description
Potassium sulfate (K2SO4) is a kind of chemical compounds that is commonly used in agriculture. The dominant application of potassium sulfate is as a fertilizer, which is commonly applied to offer both potassium and sulfur, thus improving the quality and yield of crops growing in soils that lack an adequate supply of this essential elements. Besides, the crude potassium sulfate is sometimes employed in the production of glass. It also has applications in other industries, which is used as a flash reducer in artillery propellant charges and as an alternative blast media similar to soda in the process of soda blasting.

potassium sulfate powder
Chemical properties
Potassium sulfate,K2804, also known as salt of Lemery and arcanite, is a colorless crystalline solid that melts at 1072°C(1960 OF). It is soluble in water,but insoluble in alcohol. Potassium sulfate is used in manufacturing glass, aluminum, fertilizers, and in medicine.
Chemical properties
Potassium sulfate, sulfate of potash, K2SO4, white solid, soluble. Common constituent of potassium salt minerals.
Physical properties
Colorless or white crystals or white granules or powder; rhombohedral structure; bitter taste; density 2.66 g/cm3; melts at 1,069°C; vaporizes at 1,689°C; moderately soluble in water, 12 g/100mL at 25°C and 24g/100mL at 100°C; slightly soluble in glycerol; insoluble in alcohol, acetone, and carbon disulfide.
Occurrence
Potassium and sodium sulfates and their double sulfates with calcium and magnesium occur naturally in various salt lakes. Potassium sulfate also occurs in certain volcanic lava. Its double salt with magnesium occurs in nature, as the mineral langbeinite.
Potassium sulfate is used in fertilizers as a source of potassium and sulfur, both of which are essential elements for plant growth. Either in sim-ple form or as a double salt with magnesium sulfate, potassium sulfate is one of the most widely consumed potassium salts in agricultural applications. It is preferred over potassium chloride for certain types of crops; such as, tobac-co, citrus, and other chloride-sensitive crops. Some other applications include making gypsum cements; to make potassium alum; in the analysis of Kjeldahl nitrogen; and in medicine.
The Uses of Potassium sulfate
potassium sulfate is a reagent in cosmetics. Potassium sulfate is an inorganic salt with a primary function as a viscosity-increasing agent.
The Uses of Potassium sulfate
Technical grades are used in fertilizers for manufacture of potassium alum, potassium carbonate and glass; the reagent grade is used in the Kjeldahl determination of nitrogen.
The Uses of Potassium sulfate
Potassium Sulfate is a flavoring agent that occurs naturally, consisting of colorless or white crystals or crystalline powder having a bitter, saline taste. it is prepared by the neutralization of sulfuric acid with potassium hydroxide or potassium carbonate.
Indications
Potassium is used to regulate hypokalemia as a primary condition or secondary to other medical conditions.
What are the applications of Application
Potassium sulfate is a standard laboratory reagent useful in an array of research applications
Definition
Potassium sulfate is a potassium salt and an inorganic potassium salt. It is a white crystalline powder, K2SO4, soluble inwater and insoluble in ethanol;rhombic or hexagonal; r.d. 2.66; m.p.1069°C. It occurs naturally assch nite (Strassfurt deposits) and inlake brines, from which it is separatedby fractional crystallization. Ithas also been produced by the Hargreavesprocess, which involves theoxidation of potassium chloride withsulphuric acid. In the laboratory itmay be obtained by the reaction ofeither potassium hydroxide or potassiumcarbonate with sulphuric acid.Potassium sulphate is used in cements,in glass manufacture, as a food additive, and as a fertilizer(source of K+) for chloride-sensitiveplants, such as tobacco and citrus.
Production Methods
Potassium sulfate is produced by various methods, selection of process depending on availability and cost of raw materials.
The salt may be obtained from its naturally occurring mineral, langbeinite,K2SO4?2MgSO4. The ore first is crushed and washed with water to separate sodium chloride. After that, magnetite is separated from the washed langbei-nite by magnetic separation. After the separation of these two major impuri-ties, the purified double salt is treated with an aqueous solution of potassium chloride to obtain potassium sulfate:
K2SO4?2MgSO4 + 4KCl →3K2SO4+ 2MgCl2
The solution is filtered to remove insoluble residues and the products are separated from their aqueous mixture by crystallization.
Potassium sulfate also is produced from the mineral kieserite, MgSO4?H2O by treatment with potassium chloride. The intermediate double salt obtained reacts further with potassium chloride to form potassium sulfate:MgSO4?H2O + 2KCl + 4H2O →K2SO4?MgSO4?6H2O + MgCl2
K2SO4?MgSO4?6H2O + 2KCl →2K2SO4+ MgCl2
Potassium sulfate is separated from the more soluble magnesium chloride by crystallization.
Also, potassium sulfate can be made by two other processes in which no naturally occurring mineral is employed. In the Mannheim process, the salt is produced by action of sulfuric acid on potassium chloride:2KCl + H2SO4→K2SO4+ 2HCl
In Hargreaves process, which is a slight variation of the Mannheim method, potassium sulfate is made by heating a mixture of potassium chlo-ride, sulfur dioxide, air and water:4KCl + 2SO2+ 2H2O + o2→2K2SO4+ 4HCl.
General Description
Potassium sulfate is an inorganic salt that can be prepared by reacting phosphogypsum and potassium chloride. It forms needlelike mullite particles on heating with aluminum sulfate (Al2(SO4)3) and silicon dioxide (SiO2). Its application to the soil has been reported to minimize the bronzing of rice plants. Its surface integration growth kinetics has been obtained in the temperature range of 20-50°C. The theoretical heat capacity curve of potassium sulfate in vapor phase has been obtained.
Flammability and Explosibility
Non flammable
Safety Profile
Moderately toxic to humans by ingestion. Moderately toxic experimentally by subcutaneous route. Swallowing large doses causes severe gastrointestinal tract effects. When heated to decomposition it emits toxic fumes of K2O and SOx. See also SULFATES.
Metabolism
Not Available
Purification Methods
Potassium sulfate [7778-80-5] M 174.3, m 1069o, d 4 2.67 It crystallised from distilled water (4mL/g at 20o; 8mL/g at 100o) between 100o and 0o.
References
https://en.wikipedia.org/wiki/Potassium_sulfate
http://www.cropnutrition.com/potassium-sulfate
http://study.com/academy/lesson/what-is-potassium-sulfate-structure-uses-formula.html
Properties of Potassium sulfate
| Melting point: | 1067°C |
| Boiling point: | 1689°C |
| Density | 2.66 |
| Flash point: | 1689°C |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | H2O: 0.5 M at 20 °C, clear, colorless |
| form | Very Fine Crystals or Powder |
| color | White |
| Specific Gravity | 2.662 |
| PH Range | ~7 |
| PH | 5.5-7.5 (25℃, 0.5M in H2O) |
| Odor | Odorless |
| Water Solubility | 110 g/L (20 ºC) |
| λmax | λ: 260 nm Amax: 0.01 λ: 280 nm Amax: 0.01 |
| Merck | 14,7674 |
| Dielectric constant | 5.9(0.0℃) |
| Stability: | Stable. |
| CAS DataBase Reference | 7778-80-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Potassium sulfate(7778-80-5) |
| EPA Substance Registry System | Potassium sulfate (7778-80-5) |
Safety information for Potassium sulfate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P262:Do not get in eyes, on skin, or on clothing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Potassium sulfate
| InChIKey | OTYBMLCTZGSZBG-UHFFFAOYSA-L |
Potassium sulfate manufacturer
Gujarat Kisan Fertilizer Co.
Aashi Chem
Fertinagro India Private Limited
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Potassium sulfate 99%View Details
Potassium sulfate 99%View Details -
 Potassium sulfate 98%View Details
Potassium sulfate 98%View Details -
 Potassium sulfate 99%View Details
Potassium sulfate 99%View Details -
 Potassium sulfate, For ACS analysis CAS 7778-80-5View Details
Potassium sulfate, For ACS analysis CAS 7778-80-5View Details
7778-80-5 -
 Potassium sulfate, For ACS analysis CAS 7778-80-5View Details
Potassium sulfate, For ACS analysis CAS 7778-80-5View Details
7778-80-5 -
 Potassium sulfate CAS 7778-80-5View Details
Potassium sulfate CAS 7778-80-5View Details
7778-80-5 -
 Potassium sulfate CAS 7778-80-5View Details
Potassium sulfate CAS 7778-80-5View Details
7778-80-5 -
 Potassium sulfate, 99% 99%View Details
Potassium sulfate, 99% 99%View Details
