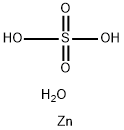
Ferrous sulfate heptahydrate
Synonym(s):o-Phenanthroline ferrous sulfate complex;1,10-Phenanthroline iron(II) sulfate complex;Ferrous sulfate heptahydrate;Iron vitriol;Iron(II) sulfate heptahydrate
- CAS NO.:7782-63-0
- Empirical Formula: FeH14O11S
- Molecular Weight: 278.01
- MDL number: MFCD00036428
- EINECS: 616-510-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-23 08:41:34
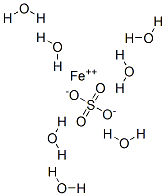
What is Ferrous sulfate heptahydrate?
Description
§184.1315(a) Ferrous sulfate heptahydrate (iron (II) sulfate heptahydrate, FeS04-7H2O) is prepared by the action of sulfuric acid on iron. It occurs as pale, blueish-green crystals or granules. Progressive heating of ferrous sulfate heptahydrate produces ferrous sulfate (dried). Ferrous sulfate (dried) consists primarily of ferrous sulfate monohydrate (CAS No. 17375-41-6) with varying amounts of ferrous sulfate tetrahydrate (CAS No. 20908-72-9) and occurs as a grayish-white to buff-colored powder.
Chemical properties
light blue or light blue-green solid
Chemical properties
Ferrous sulfate is a greenish or yellowish solid in fine or lumpy crystals.
The Uses of Ferrous sulfate heptahydrate
Used in quantitative analysis of nitrates .
The Uses of Ferrous sulfate heptahydrate
In manufacture of Fe, Fe Compounds, other sulfates; in Fe electroplating baths; in fertilizer; as food and feed supplement; in radiation dosimeters; as reducing agent in chemical processes; as wood preservative; as weed-killer; in prevention of chlorosis in plants; in other pesticides; in writing ink; in process engraving and lithography; as dye for leather; in etching aluminum; in water treatment; in qualitative analysis ("brown ring" test for nitrates); as polymerization catalyst.
The Uses of Ferrous sulfate heptahydrate
Iron(II) sulfate heptahydrate is used as a precursor to prepare other iron compounds such as a lawn conditioner and a mordant for wool dyeing. It is actively used in the manufacture of ink including iron gall ink. As a reducing agent, it participates in the reduction of chromate in cement. It is also used in industrial water treatment plants to remove phosphate. It is also used in the gold refining process to precipitate metallic gold. Woodworkers use aqueous solutions of ferrous sulfate to color maple wood a silvery hue. It finds application in the treatment of iron chlorosis, which arises due to deficiency of iron in horticulture.
What are the applications of Application
Ferrous Sulfate (Iron II Sulfate) Heptahydrate is A reducing agent with many other applications
Definition
ChEBI: A hydrate that is the heptahydrate form of iron(2+) sulfate. It is used as a source of iron in the treatment of iron-deficiency anaemia (generally in liquid-dosage treatments; for solid-dosage treatments, the monohydrate is normally used).
General Description
A greenish or yellow-brown crystalline solid. Density 15.0 lb /gal. Melts at 64°C and loses the seven waters of hydration at 90°C. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Used for water or sewage treatment, as a fertilizer ingredient.
Air & Water Reactions
Water soluble
Reactivity Profile
Ferrous sulfate heptahydrate is a weak reducing agent.
Health Hazard
INGESTION: abdominal pain, retching, diarrhea, dehydration, shock, pallor, cyanosis, rapid or weak pulse, shallow respiration, low blood pressure.
Safety Profile
Poison by intravenous, intraperitoneal, and subcutaneous routes. Moderately toxic by ingestion and rectal routes. Mutation data reported. When heated to decomposition it emits toxic fumes of SOx.
Potential Exposure
It is used as a fertilizer, food or feed additive; and in herbicides; process engraving; dyeing, and water treatment. A byproduct of various chemical and metal treating operations.
Purification Methods
Crystallise the sulfate from 0.4M H2SO4, or precipitate it from an aqueous solution with EtOH. It is efflorescent in dry air, and is converted to the tetrahydrate at 57o, then to the monohydrate at 65o (or by heating the heptahydrate in a vacuum at 140o). It forms a brown-black complex, FeSO4.NO, with nitric oxide and is used in a qualitative test for nitrates (“brown ring” test).
Incompatibilities
Aqueous solution is acidic. Contact with alkalies form iron. Keep away from alkalies, soluble carbo nates; gold and silver salts; lead acetate; lime water, potassium iodide; potassium and sodium tartrate; sodium borate; tannin.
Properties of Ferrous sulfate heptahydrate
| Melting point: | 64 °C |
| Density | 1.898 g/mL at 25 °C (lit.) |
| vapor pressure | 14.6 mm Hg ( 25 °C) |
| storage temp. | Store at +15°C to +25°C. |
| solubility | 25.6 g/100 mL (20°C) |
| form | Solution |
| color | Slightly greenish to blue |
| Specific Gravity | 1.898 |
| PH | 3.0-4.0 (25℃, 50mg/mL in H2O) |
| Odor | pale bluish-grn. cryst. or gran., odorless |
| Water Solubility | 25.6 g/100 mL (20 ºC) |
| Sensitive | Air Sensitive & Hygroscopic |
| Merck | 14,4057 |
| Exposure limits | ACGIH: TWA 1 mg/m3 NIOSH: TWA 1 mg/m3 |
| Stability: | Stable. Substances to be avoided include strong oxidizing agents. Air and moisture sensitive. |
| CAS DataBase Reference | 7782-63-0(CAS DataBase Reference) |
| EPA Substance Registry System | Ferrous sulfate heptahydrate (7782-63-0) |
Safety information for Ferrous sulfate heptahydrate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Ferrous sulfate heptahydrate
| InChIKey | SURQXAFEQWPFPV-UHFFFAOYSA-L |
Ferrous sulfate heptahydrate manufacturer
JSK Chemicals
ASM Organics
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Ferrous sulfate heptahydrate 98%View Details
Ferrous sulfate heptahydrate 98%View Details -
 Ferrous sulfate heptahydrate 99%View Details
Ferrous sulfate heptahydrate 99%View Details -
 Iron(II) sulfate heptahydrate, For ACS analysis CAS 7782-63-0View Details
Iron(II) sulfate heptahydrate, For ACS analysis CAS 7782-63-0View Details
7782-63-0 -
 Iron(II) sulfate heptahydrate, For ACS analysis CAS 7782-63-0View Details
Iron(II) sulfate heptahydrate, For ACS analysis CAS 7782-63-0View Details
7782-63-0 -
 Iron(II) sulfate heptahydrate CAS 7782-63-0View Details
Iron(II) sulfate heptahydrate CAS 7782-63-0View Details
7782-63-0 -
 Iron(II) sulfate heptahydrate CAS 7782-63-0View Details
Iron(II) sulfate heptahydrate CAS 7782-63-0View Details
7782-63-0 -
 Iron(II) sulfate heptahydrate, 98% 99%View Details
Iron(II) sulfate heptahydrate, 98% 99%View Details -
 Ferrous Sulphate Heptahydrate for tissue culture CAS 7782-63-0View Details
Ferrous Sulphate Heptahydrate for tissue culture CAS 7782-63-0View Details
7782-63-0
