Silver sulfate
Synonym(s):Disilver sulfate;Silver Sulfate;Sulfuric acid disilver(I) salt
- CAS NO.:10294-26-5
- Empirical Formula: Ag2O4S
- Molecular Weight: 311.8
- MDL number: MFCD00003407
- EINECS: 233-653-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:53
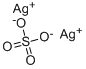
What is Silver sulfate?
Chemical properties
Small crystals or powder, colorless and shiny. Contains approximately 69% silver and turns gray when exposed to light. Melts at 652°C and decomposes at 1,085°C. Partially dissolves in water and completely dissolves in solutions containing ammonium hydroxide, nitric acid, sulfuric acid, and hot water. Does not dissolve in alcohol. Its solubility in pure water is low, but it increases when the pH of the solution decreases. When the concentration of H+ ions is high enough, it can dissolve significantly.
The Uses of Silver sulfate
Silver sulfate is used as a catalyst to oxidize long chain aliphatic hydrocarbons in the determination of chemical oxygen demand (COD).
The Uses of Silver sulfate
Silver sulfate is used as a catalyst to oxidize long chain aliphatic hydrocarbons in the determination of chemical oxygen demand (COD). It serves as a catalyst in wastewater treatment and aids in the production of nanostructured metallic layers beneath Langmuir monolayers.
What are the applications of Application
Silver sulfate is an ionic compound of silver employed in silver plating
Preparation
Silver sulfate is precipitated by adding sulfuric acid to a solution of silvernitrate:2Ag+(aq) + SO42- (aq) → Ag2SO4 (s) The precipitate is washed with hot water and preparation is under ruby red illumination.
What are the applications of Application
Silver sulfate may be employed in the following studies:
Iodination reagent in combination with iodine for the synthesis of iododerivatives.
Synthesis of iodinated uredines.
General Description
Silver sulfate is an odorless white to gray solid. Sinks and mixes with water. (USCG, 1999)
Reactivity Profile
Silver sulfate has weak oxidizing or reducing powers. Redox reactions can however still occur. The majority of compounds in this class are slightly soluble or insoluble in water. If soluble in water, then the solutions are usually neither strongly acidic nor strongly basic. These compounds are not water-reactive.
Hazard
Highly toxic.
Health Hazard
Contact with eyes causes irritation. If continued for a long period, ingestion or inhalation of silver compounds can cause permanent discoloration of the skin (argyria).
Purification Methods
Crystallise the sulfate form hot conc H2SO4 containing a trace of HNO3, and dilute with H2O while being strongly cooled. The precipitate is filtered off, washed with H2O and dried at 120o. Its solubility in H2O is 0.8% at 17o, and 1.46% at 100o. Store it in the dark. [Glemser & Sauer in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol II p 1042 1965.]
Properties of Silver sulfate
| Melting point: | 652 °C (lit.) |
| Boiling point: | 1085 °C |
| Density | 5.45 |
| Flash point: | 1085°C |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | 8g/l |
| form | Solid |
| Specific Gravity | 5.45 |
| color | White to off-white |
| PH | 5-6 (5g/l, H2O, 25℃) |
| Water Solubility | 7.4 g/L (20 ºC) |
| Sensitive | Light Sensitive |
| Merck | 14,8529 |
| Solubility Product Constant (Ksp) | pKsp: 4.92 |
| Exposure limits | ACGIH: TWA 0.01 mg/m3 NIOSH: IDLH 10 mg/m3; TWA 0.01 mg/m3 |
| Stability: | Stability Stable, but light-sensitive. |
| CAS DataBase Reference | 10294-26-5(CAS DataBase Reference) |
| EPA Substance Registry System | Silver sulfate (10294-26-5) |
Safety information for Silver sulfate
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Environment GHS09 |
| GHS Hazard Statements |
H318:Serious eye damage/eye irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P391:Collect spillage. Hazardous to the aquatic environment P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P501:Dispose of contents/container to..… |
Computed Descriptors for Silver sulfate
| InChIKey | YPNVIBVEFVRZPJ-UHFFFAOYSA-L |
Silver sulfate manufacturer
JSK Chemicals
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 Silver Sulphate 98%View Details
Silver Sulphate 98%View Details -
 Silver sulfate 99%View Details
Silver sulfate 99%View Details -
 Silver sulfate CAS 10294-26-5View Details
Silver sulfate CAS 10294-26-5View Details
10294-26-5 -
 Silver sulfate CAS 10294-26-5View Details
Silver sulfate CAS 10294-26-5View Details
10294-26-5 -
 Silver sulfate CAS 10294-26-5View Details
Silver sulfate CAS 10294-26-5View Details
10294-26-5 -
 Silver sulfate CAS 10294-26-5View Details
Silver sulfate CAS 10294-26-5View Details
10294-26-5 -
 Silver sulfate CAS 10294-26-5View Details
Silver sulfate CAS 10294-26-5View Details
10294-26-5 -
 SILVER SULPHATE AR STCView Details
SILVER SULPHATE AR STCView Details
10294-26-5
