CERIC SULFATE
Synonym(s):Ceric sulfate;Cerium disulfate
- CAS NO.:13590-82-4
- Empirical Formula: CeH2O4S
- Molecular Weight: 238.19
- MDL number: MFCD00148852
- EINECS: 237-029-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
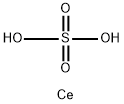
What is CERIC SULFATE?
Chemical properties
yellow odourless solid
Physical properties
White crystalline powder; orthogonal crystal system; the tetrahydrate is a yellow-to-orange powder which, on heating at 180°C, loses all molecules of water; density of tetrahydrate 3.91 g/cm3; anhydrous salt decomposes at 350°C forming CeOSO4; soluble in water (decomposes); soluble in dilute H2SO4 and other concentrated mineral acids.
The Uses of CERIC SULFATE
calibrated against sodium oxalate, traceable to NIST SRM
The Uses of CERIC SULFATE
Cerium(IV) sulfate is a redox indicator and is used in analytical chemistry for redox titrations. In ionized form, ceric ion acts as a strong oxidizer under acidic conditions. It is also used to prepare spherical-like nanoparticles CeO2, nanoparticles for NO reductions' reactions.
What are the applications of Application
Cerium(IV) sulfate is an oxidizing agent used in analytical chemistry
Preparation
Cerium(IV) sulfate is prepared by heating cerium(IV) oxide, CeO2 with concentrated H2SO4. Also it may be obtained by the reaction of H2SO4 with cerium carbonate:
Ce(CO3)2 + 2H2SO4 + H2O → Ce(SO4)2?4H2O + 2CO2.
General Description
3S - Safe, Smart, Secure
An intelligent packaging solution protected with originality seal.
Reagent information is transferred via RFID from reagent bottle to titrator.
3S reagents are compatible only with the Metrohm Omnis titration platform.
Please see here SigmaAldrich.com/3S
Flammability and Explosibility
Not classified
Safety Profile
Experimental reproductive effects. When heated to decomposition it emits acrid smoke and irritating fumes.
Properties of CERIC SULFATE
| Density | 3.01 g/mL at 25 °C(lit.) |
| vapor pressure | 0.001Pa at 20℃ |
| storage temp. | Inert atmosphere,Room Temperature |
| form | Liquid |
| color | Light yellow |
| Water Solubility | Soluble in water and dilute sulfuric acid. |
| Sensitive | Hygroscopic |
| Merck | 14,1990 |
| CAS DataBase Reference | 13590-82-4(CAS DataBase Reference) |
| EPA Substance Registry System | Sulfuric acid, cerium(4+) salt (2:1) (13590-82-4) |
Safety information for CERIC SULFATE
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Environment GHS09 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for CERIC SULFATE
CERIC SULFATE manufacturer
Star Earth Minerals Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Ceric(IV) sulphate 98%View Details
Ceric(IV) sulphate 98%View Details
13590-82-4 -
 Ceric sulfate 99%View Details
Ceric sulfate 99%View Details -
 Cerium(IV) sulfate CAS 13590-82-4View Details
Cerium(IV) sulfate CAS 13590-82-4View Details
13590-82-4 -
 Ceric Sulphate 0.1N Solution CAS 13590-82-4View Details
Ceric Sulphate 0.1N Solution CAS 13590-82-4View Details
13590-82-4 -
 Ceric sulphate (N/10) solution CAS 13590-82-4View Details
Ceric sulphate (N/10) solution CAS 13590-82-4View Details
13590-82-4 -
 Cerium(IV) sulphate, GR 99.9% CAS 13590-82-4View Details
Cerium(IV) sulphate, GR 99.9% CAS 13590-82-4View Details
13590-82-4 -
 Ceric sulfate 95% CAS 13590-82-4View Details
Ceric sulfate 95% CAS 13590-82-4View Details
13590-82-4 -
 Cerium(IV) sulfate solution CASView Details
Cerium(IV) sulfate solution CASView Details