Potassium borohydride
Synonym(s):Potassium borohydride;Potassium tetrahydroborate;Potassium tetrahydroborate, Potassium boranate
- CAS NO.:13762-51-1
- Empirical Formula: BH4K
- Molecular Weight: 53.94
- MDL number: MFCD00011396
- EINECS: 237-360-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
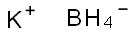
What is Potassium borohydride?
Chemical properties
White, crystalline powder. Stable in moist and dry air, stable in vacuum to 500C, decomposed by acids with evolution of hydrogen. Partially soluble in water and ammonia; insoluble in ethers and hydrocarbons.
The Uses of Potassium borohydride
Source of hydrogen, reducing agent for aldehydes, ketones, acid chlorides; foaming agent for plasticsPotassium borohydride is used as a selective reducing agent for ketones, aldehydes and acid chlorides. It is used as a precursor in the production of chloramphenicol, vitamin A, thiopenicol, atropine and scopolamine. It is used to prepare high dispersive copper nanoparticles by the chemical reduction method using potassium borohydride as a reducing agent.
The Uses of Potassium borohydride
Reducing agent; source of H-.
What are the applications of Application
Potassium borohydride is a chemical used in synthesis as a selective reducing agent
General Description
Potassium borohydride is a white crystalline or powdered solid. When exposed to water Potassium borohydride may react violently and start a fire. Potassium borohydride is toxic by ingestion. Potassium borohydride is used to make other chemicals.
Air & Water Reactions
Highly flammable. When exposed to water Potassium borohydride may react violently and start a fire. Produce flammable gases on contact with water. May ignite on contact with water or moist air.
Reactivity Profile
A reducing agent. Reacts rapidly and dangerously with oxygen and with other oxidizing agents, even weak ones. Thus, they are likely to ignite on contact with alcohols. Hydrides are incompatible with acids, alcohols, amines, and aldehydes.
Hazard
Flammable, dangerous fire risk. Toxic by ingestion.
Health Hazard
Inhalation or contact with vapors, substance or decomposition products may cause severe injury or death. May produce corrosive solutions on contact with water. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution.
Fire Hazard
Produce flammable gases on contact with water. May ignite on contact with water or moist air. Some react vigorously or explosively on contact with water. May be ignited by heat, sparks or flames. May re-ignite after fire is extinguished. Some are transported in highly flammable liquids. Runoff may create fire or explosion hazard.
Safety Profile
Poison by ingestion. Burns quietly in air. When heated to decomposition it emits toxic fumes of K2O. See also BORON COMPOUNDS and HYDRIDES.
Purification Methods
Crystallise it from liquid ammonia. It is slowly hydrolysed by H2O. Its solubility at ~20o in H2O or liquid NH3 is 20%, in MeOH it is 0.7%, in Me2NCHO it is 15% and in MeOH/H2O (1:4) it is 13%. [Jons & Wallbridge Progr Inorg Chem 11 99-231 1970.]
Properties of Potassium borohydride
| Melting point: | 500 °C (dec.) (lit.) |
| Density | 1.18 g/mL at 25 °C (lit.) |
| refractive index | 1.494 |
| storage temp. | water-free area |
| solubility | soluble in H2O |
| form | Powder |
| color | White |
| Specific Gravity | 1.178 |
| Water Solubility | 190 g/L (25 ºC) |
| Sensitive | Moisture Sensitive |
| Merck | 14,7616 |
| CAS DataBase Reference | 13762-51-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Potassium tetrahydroborate(13762-51-1) |
| EPA Substance Registry System | Borate(1-), tetrahydro-, potassium (1:1) (13762-51-1) |
Safety information for Potassium borohydride
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H260:Substances And Mixtures Which, In Contact With Water,Emit Flammable Gases H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P231+P232:Handle under inert gas. Protect from moisture. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. |
Computed Descriptors for Potassium borohydride
| InChIKey | ICRGAIPBTSPUEX-UHFFFAOYSA-N |
Potassium borohydride manufacturer
Ronak Alkalies And Chemicals Private Limited
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
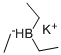
You may like
-
 Potassium borohydride CAS 13762-51-1View Details
Potassium borohydride CAS 13762-51-1View Details
13762-51-1 -
 Potassium borohydride CAS 13762-51-1View Details
Potassium borohydride CAS 13762-51-1View Details
13762-51-1 -
 Potassium borohydride CAS 13762-51-1View Details
Potassium borohydride CAS 13762-51-1View Details
13762-51-1 -
 Potassium Borohydride 98% CASView Details
Potassium Borohydride 98% CASView Details -
 Potassium Borohydride 97% Min CAS 13762-51-1View Details
Potassium Borohydride 97% Min CAS 13762-51-1View Details
13762-51-1 -
 POTASSIUM BOROHYDRIDE CAS No.: 13762-51-1View Details
POTASSIUM BOROHYDRIDE CAS No.: 13762-51-1View Details
13762-51-1 -
 RAW cas 13762-51-1 Potassium borohydride APIView Details
RAW cas 13762-51-1 Potassium borohydride APIView Details
13762-51-1 -
 Potassium Borohydride (13762-51-10), 50Kg bag, For LaboratoryView Details
Potassium Borohydride (13762-51-10), 50Kg bag, For LaboratoryView Details
13762-51-1
