Potassium persulfate
Synonym(s):KPS;Potassium peroxodisulfate;Potassium persulfate
- CAS NO.:7727-21-1
- Empirical Formula: K2O8S2
- Molecular Weight: 270.32
- MDL number: MFCD03457781
- EINECS: 231-781-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:59
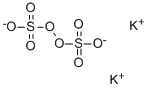
What is Potassium persulfate?
Description
Potassium persulfate, is composed of white crystals that are soluble in water, and it decomposes below 212°F (100°C). Potassium persulfate is a dangerous fire risk in contact with organic materials. It is a strong oxidizing agent and an irritant, with a four-digit UN identification number of 1492. The primary uses are in bleaching, as an oxidizing agent, as an antiseptic, as a polymerization promoter, and in the manufacture of pharmaceuticals.
Chemical properties
colourless odourless crystals or white powder
Chemical properties
Potassium persulfate is a colorless or white, odorless crystalline material.
Physical properties
Colorless or white crystals; triclinic structure; density 2.477 g/cm3; stable in solid crystalline form; decomposes on heating, evolving oxygen; completely decomposes at about 100°C; sparingly soluble in cold water 1.75 g/100mL at 0°C; moderately soluble at ordinary temperature, 5.29 g/100 mL at 20°C;aqueous solution acidic and unstable, decomposing slowly at room temperature and more rapidly when the solution is warmed; insoluble in alcohol.
The Uses of Potassium persulfate
Potassium persulfate?is a powerful oxidant, commonly used to initiate polymerizations. Potassium Persulphate used for bleaching and textile desizing, as an oxidizing agent and antiseptic, in purification of ammonium sulfate, and in the manufacture of soap and pharmaceuticals. It is also used as al laboratory oxidant and photography chemical. It is a food additive.
The Uses of Potassium persulfate
Bleaching fabrics, soaps; in photography under the name Anthion to remove last traces of thiosulfate from plates and paper; in analytical chemistry.
The Uses of Potassium persulfate
Potassium persulfate is used to initiate the polymerization of styrene to form monodispersed, surfactant-free polystyrene spheres. Potassium persulfate is used as an oxidant to generate 2,2-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical cation to measure the antioxidant activities of natural compounds.
What are the applications of Application
Potassium persulfate is a laboratory oxidant and photography chemical
Preparation
Potassium persulfate can be prepared by electrolysis of a mixture of potassium sulfate and potassium hydrogen sulfate at a high current density:
2KHSO4→K2S2O8+ H2
Also, the compound can be prepared by adding potassium hydrogen sulfate,KHSOto an electrolyzed solution of ammonium hydrogen sulfate, NH4HSO4.
General Description
A white crystalline solid. Specific gravity 2.477. Decomposes below 100°C.
Air & Water Reactions
Water soluble. Slowly decomposed by water. The salt rapidly liberates oxygen when heated, and especially so when wet.
Reactivity Profile
Potassium persulfate is an oxidizing agent. Noncombustible but accelerates the burning of combustible material. Potassium persulfate plus a little potassium hydroxide and water released sufficient heat and oxygen to ignite a polythene (polyethylene) liner in a container. [MCA Case History 1155. 1955].
Hazard
Strong irritant and oxidizing agent. Fire risk in contact with organic materials.
Health Hazard
Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.
Flammability and Explosibility
Non flammable
Agricultural Uses
Potassium sulphate, also called sulphate of potash, is a
white crystalline material, moderately hygroscopic,
available in fine, granular and semi-granular forms. It
contains 48 to 54% potassium (as K2O) and supplies 17 to
20 % of sulphate. Chloride-sensitive crops like tobacco,
grapes and potato require chloride-free potassium
fertilizers. Therefore, these crops are fertilized with
potassium sulphate, although this is more expensive than
potassium chloride. These three crops, being major
crops, account for about 7% of the total potash
consumption. For best results, potassium sulphate should
contain at least 50 % potash by weight.
Potassium sulphate occurs in nature as 'langbeinite' ,
a double sulphate of potassium and magnesium
(K2SO4?2MgSO4) and made from burkeite
(Na2CO3?2Na2SO4), kainite (KCl?MgS04?3H2O) or
potassium chloride (KCl) as follows:
When applied to soil, potassium ion from the watersoluble
potassium sulphate is retained in the soil colloids
and not easily leached out. This makes potassium
sulphate an excellent fertilizer, useful for all soils and
crops while sowing or before sowing. It is also a safe
ingredient of powdered, mixed fertilizers.
Safety Profile
Moderately toxic by ingestion. An irritant and allergen. A powerful oxidtzer. Flammable when exposed to heat or by chemical reaction. Can react with reducing materials. It liberates oxygen above 100' when dry or @ about 50' when in solution. When heated to decomposition it emits highly toxic fumes of SOx,, S2O8, and K2O.
Potential Exposure
Potassium persulfate is used as a bleaching and oxidizing agent; it is used in redox polymeri- zation catalysts; in the defiberizing of wet strength paper and in the desizing of textiles. Soluble in water.
Shipping
UN1492 Potassium persulfate, Hazard Class: 5.1; Labels: 5.1-Oxidizer.
Purification Methods
Crystallise the persulfate twice from distilled water (10mL/g) and dry it at 50o in a vacuum desiccator. Its solubility in H2O is 1.6% at 0o, 4.5% at 20o, and 7.2% at 30o. An aqueous solution decomposes on long standing with evolution of O2 and formation of KHSO4. It is a powerful oxidising agent. Store it at ~10o. [Fehér in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 390 1963.]
Incompatibilities
A strong oxidizer. Incompatible with combustible, organic or other readily oxidizable materials; sulfur, metallic dusts, such as aluminum dust; chlorates and perchlorates. Attacks chemically active metals. Keep away from moisture.
Waste Disposal
Use large volumes of reducing agents (bisulfites, e.g.). Neutralize with soda ash and drain into sewer with abundant water.
Properties of Potassium persulfate
| Melting point: | 1067 °C |
| Boiling point: | 1689 °C |
| Density | 2.47 |
| vapor density | 9.3 (vs air) |
| vapor pressure | 0Pa at 25℃ |
| storage temp. | Store at +5°C to +30°C. |
| solubility | H2O: 0.5 M at 20 °C, clear, colorless |
| form | Solid |
| color | White |
| Specific Gravity | 2.477 |
| PH Range | 2.5 - 4.5 |
| PH | 3.2 (50g/l, H2O, 20℃) |
| Odor | Odorless |
| Water Solubility | 5 g/100 mL (20 ºC) |
| Merck | 14,7656 |
| Exposure limits | ACGIH: TWA 0.1 mg/m3 |
| Stability: | Stable. Strong oxidizer. Incompatible with strong reducing agents, organic materials, combustible materials. |
| CAS DataBase Reference | 7727-21-1(CAS DataBase Reference) |
| EPA Substance Registry System | Potassium persulfate (7727-21-1) |
Safety information for Potassium persulfate
| Signal word | Danger |
| Pictogram(s) |
 Flame Over Circle Oxidizers GHS03  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H272:Oxidising liquids;Oxidising solids H302:Acute toxicity,oral H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H334:Sensitisation, respiratory H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Potassium persulfate
| InChIKey | USHAGKDGDHPEEY-UHFFFAOYSA-L |
Potassium persulfate manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
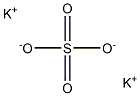

You may like
-
 Potassium persulfate 98%View Details
Potassium persulfate 98%View Details -
 Potassium peroxydisulfate CAS 7727-21-1View Details
Potassium peroxydisulfate CAS 7727-21-1View Details
7727-21-1 -
 Potassium PersulfateView Details
Potassium PersulfateView Details
7727-21-1 -
 Potassium Persulphate For Industrial, Grade: IndutrialView Details
Potassium Persulphate For Industrial, Grade: IndutrialView Details
7727-21-1 -
 Potassium Persulphate ChemicalView Details
Potassium Persulphate ChemicalView Details
7727-21-1 -
 Potassium PersulfateView Details
Potassium PersulfateView Details
7727-21-1 -
 Potassium Persulfate powder, 25Kg bagView Details
Potassium Persulfate powder, 25Kg bagView Details
7727-21-1 -
 Potassium Persulfate ( PPS), >99%, 25kg BagView Details
Potassium Persulfate ( PPS), >99%, 25kg BagView Details
7727-21-1
