Potassium
Synonym(s):K 006100;Potassium chloride solution
- CAS NO.:7440-09-7
- Empirical Formula: K
- Molecular Weight: 39.1
- MDL number: MFCD00133776
- EINECS: 231-119-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13

What is Potassium?
Absorption
When taken orally from a dietary source, potassium is mainly absorbed via passive diffusion in the small intestine. Approximately 90% of potassium is absorbed, and maintains concentrations both inside and outside cells. The kidneys can adapt to variable potassium intake in healthy individuals, but a minimum of 5 mmol (about 195 mg) dietary potassium is measured to be excreted in the urine.
Some studies have measured the absorption various forms of potassium from dietary supplements. Results from a clinical trial in 2016 showed that potassium gluconate supplements are 94% absorbed, which is similar to the absorption rate from potatoes. An older study advised that liquid forms of potassium are absorbed a few hours post-administration. Enteric coated tablets of potassium chloride are not absorbed as rapidly as liquid forms, due to their delayed release design.
Toxicity
The oral LD50 of potassium chloride in rats is 2600 mg/kg.
Overdose information
An overdose of potassium may result in hyperkalemia, and in some cases, death due to various causes. Signs and symptoms of an overdose of potassium are mainly cardiovascular, neurological and musculoskeletal in nature. Arrhythmia, changes in cardiac conduction, including astystole, bradycardia, heart block, ventral fibrillation, and ventricular tachycardia may occur. In addition, hypotension may also occur along with cardiac ECG changes. Muscular weakness and respiratory muscle paralysis may occur, in addition to paresthesia. In case of an overdose, discontinue potassium administration, reduce the dose, and monitor fluid levels and electrolyte concentrations in addition to acid-base balance. Corrective therapy, such as insulin administration or potassium binding drugs, may be required. Offer supportive care and resuscitation as deemed necessary.
Important note regarding hyperkalemia
Normally, hyperkalemia is asymptomatic and only detected by laboratory testing (at values of 6.5-8.0 mEq/L) and ECG changes (peaked T- waves, lost P-waves, ST depression, and a prolonged QT interval). Muscle paralysis and cardiac arrest may occur in the advanced stages of hyperkalemia, at potassium concentrations of 9-12 mEq/L.
Description
Potassium has atomic number 19 and the chemical symbol K, which is derived from its Latin name kalium . Potassium was first isolated from potash, which is potassium carbonate (K2CO3). Potassium occurs in nature only in the form of its ion (K+) either dissolved in the ocean or coordinated in minerals because elemental potassium reacts violently with water . Potassium ions are essential for the human body and are also present in plants. The major use of K+ can be found in fertilisers, which contains a variety of potassium salts such as potassium chloride (KCl), potassium sulfate (K2SO4) and potassium nitrate (KNO3).
Chemical properties
Potassium is a soft silvery metal, tarnishing upon exposure to air.
Physical properties
Elemental potassium is a soft, butter-like silvery metal whose cut surface oxidizes in dryair to form a dark gray potassium superoxide (KO2) coating. KO2 is an unusual compound,in that it reacts with both water and carbon dioxide to produce oxygen gas. It appears morelike a hard wax than a metal. Its density (specific gravity) is 0.862 g/cm3, its melting point is63.25°C, and its boiling point is 760°C. It has an oxidation state of +1 and reacts explosivelywith room temperature air or water to form potassium hydroxide as follows: 2K + 2 H2O→? 2KOH + H2. This is an endothermic reaction, which means the heat generated is greatenough to ignite the liberated hydrogen gas. Potassium metal must be stored in a non-oxygen,non-aqueous environment such as kerosene or naphtha.
Isotopes
A total of 18 isotopes of potassium have been discovered so far. Just two ofthem are stable: K-39 makes up 93.2581% of potassium found in the Earth’s crust, andK-41 makes up 6.7301% of the remainder of potassium found on Earth. All the other16 potassium isotopes are unstable and radioactive with relatively short half-lives, and asthey decay, they produce beta particles. The exception is K-40, which has a half-life of1.25×109 years.
Origin of Name
Its symbol “K” is derived from the Latin word for alkali, kalium, but it is commonly called “potash” in English.
Occurrence
Potassium is the eighth most abundant element in the Earth’s crust, which contains about2.6% potassium, but not in natural elemental form. Potassium is slightly less abundant thansodium. It is found in almost all solids on Earth, in soil, and in seawater, which contains 380ppm of potassium in solution. Some of the potassium ores are sylvite, carnallite, and polyhalite. Ore deposits are found in New Mexico, California, Salt Lake in Utah, Germany, Russia,and Israel. Potassium metal is produced commercially by two processes. One is thermochemical distillation, which uses hot vapors of gaseous NaCl (sodium chloride) and KCl (potassiumchloride); the potassium is cooled and drained off as molten potassium, and the sodium chloride is discharged as a slag. The other procedure is an electrolytic process similar to that used toproduce lithium and sodium, with the exception that molten potassium chloride (which meltsat about 770°C) is used to produce potassium metal at the cathode.
History
Discovered in 1807 by Davy, who obtained it from caustic potash (KOH); this was the first metal isolated by electrolysis. The metal is the seventh most abundant and makes up about 2.4% by weight of the Earth’s crust. Most potassium minerals are insoluble and the metal is obtained from them only with great difficulty. Certain minerals, however, such as sylvite, carnallite, langbeinite, and polyhalite are found in ancient lake and sea beds and form rather extensive deposits from which potassium and its salts can readily be obtained. Potash is mined in Germany, New Mexico, California, Utah, and elsewhere. Large deposits of potash, found at a depth of some 1000 m in Saskatchewan, promise to be important in coming years. Potassium is also found in the ocean, but is present only in relatively small amounts compared to sodium. The greatest demand for potash has been in its use for fertilizers. Potassium is an essential constituent for plant growth and it is found in most soils. Potassium is never found free in nature, but is obtained by electrolysis of the hydroxide, much in the same manner as prepared by Davy. Thermal methods also are commonly used to produce potassium (such as by reduction of potassium compounds with CaC2, C, Si, or Na). It is one of the most reactive and electropositive of metals. Except for lithium, it is the lightest known metal. It is soft, easily cut with a knife, and is silvery in appearance immediately after a fresh surface is exposed. It rapidly oxidizes in air and should be preserved in a mineral oil. As with other metals of the alkali group, it decomposes in water with the evolution of hydrogen. It catches fire spontaneously on water. Potassium and its salts impart a violet color to flames. Twenty-one isotopes, one of which is an isomer, of potassium are known. Ordinary potassium is composed of three isotopes, one of which is 40K (0.0117%), a radioactive isotope with a half-life of 1.26 × 109 years. The radioactivity presents no appreciable hazard. An alloy of sodium and potassium (NaK) is used as a heat-transfer medium. Many potassium salts are of utmost importance, including the hydroxide, nitrate, carbonate, chloride, chlorate, bromide, iodide, cyanide, sulfate, chromate, and dichromate. Metallic potassium is available commercially for about $1200/ kg (98% purity) or $75/g (99.95% purity).
Characteristics
Because its outer valence electrons are at a greater distance from its nuclei, potassium ismore reactive than sodium or lithium. Even so, potassium and sodium are very similar in theirchemical reactions. Due to potassium’s high reactivity, it combines with many elements, particularly nonmetals. Like the other alkali metals in group 1, potassium is highly alkaline (caustic) with a relatively high pH value. When given the flame test, it produces a violet color.
The Uses of Potassium
In synthesis of inorganic potassium Compounds; in organic syntheses involving condensation, dehalogenation, reduction, and polymerization reactions. As heat transfer medium together with sodium: Chem. Eng. News 33, 648 (1955). Radioactive decay of 40K to 40Ar used as tool for geological dating.
The Uses of Potassium
Some of the most common compounds in 19th century photography were made with this silvery metallic element discovered by Sir Humphrey Davy in 1807. There is not enough room in this work to list all of these compounds, but the following represent a reasonable sampling.
The Uses of Potassium
Potassium is used in the manufacture ofmany reactive potassium salts, in organicsynthesis, and as a heat exchange fluid whenalloyed with sodium.
The Uses of Potassium
Liquid potassium, when mixed with liquid sodium (NaK), is an alloy used as a heatexchange substance to cool nuclear reactors. Potassium is an important reagent (something that is used in chemical reactions to analyze other substances) that forms many compounds used in chemical and industrial laboratories. It is used to manufacture both hard and soft soaps, as a bleaching agent, and where a highly caustic chemical is required. Potassium is essential to all living organisms. It is a trace element required for a healthy diet and is found in many foods. One natural source is bananas.
Background
Potassium is an essential nutrient, like Calcium and Magnesium. It was identified as a shortfall nutrient by the 2015-2020 Advisory Committee of Dietary Guidelines for Americans. Many conditions and diseases interfere with normal body potassium balance, and underconsumption of potassium is one example. Hypokalemia (low potassium) or hyperkalemia (high potassium) may result, manifesting as various signs and symptoms. Some examples of potassium-related complications include life-threatening arrhythmia, neuromuscular dysfunction, diarrhea, nausea, and vomiting.
Various pharmacological preparations have been formulated to replenish potassium. They are available in an assortment of tablet, injection, and other forms, depending on the setting and condition being treated. Potassium is often a key ingredient for intravenous fluids, given to patients in clinical settings for rehydration, nutrition, and replenishment of electrolytes. Examples of potassium formulations include potassium citrate, potassium chloride, and potassium with dextrose and sodium chloride.
Indications
General uses of potassium
Potassium is indicated to treat a variety of conditions. Firstly, it used to replenish potassium that has been depleted by conditions including but not limited to malabsorption, decreased intake, or excess sodium intake. The causes of potassium deficiency are numerous. The following indications for potassium are not comprehensive, but include the main indications for which this nutrient is used. Various products and preparations contain potassium.
Potassium chloride
Potassium chloride is one of the main preparations of potassium used in a clinical setting. The oral solution is indicated for the prevention and treatment of hypokalemia presenting with or without metabolic alkalosis, in patients who have failed conservative management with potassium-rich foods or diuretic dose titrations. The injection form of potassium chloride is indicated to replenish potassium in patients who are not feasible candidates for oral potassium. Highly concentrated potassium is intended for the treatment of potassium deficiency in fluid restricted individuals who cannot tolerate fluid volumes normally associated with injected potassium solutions that contain lower concentrations.
Finally, the extended-release tablet preparation of potassium chloride is used to treat hypokalemia with or without metabolic alkalosis, to treat digitalis intoxication, and to manage patients with hypokalemic familial periodic paralysis. It is also used in the prevention of hypokalemia in those who are at a high risk of negative clinical outcomes if hypokalemia occurs; patients on digitalis or those with cardiac arrhythmias would be at particular risk of negative outcomes.
Potassium chloride with dextrose and sodium chloride
This liquid preparation is is indicated in a clinical setting as a source of water, calories and electrolytes. Potassium acetate solution is meant as an alternative to potassium chloride, replenishing potassium and added to large volume infusion fluids for intravenous injection.
Potassium citrate
The potassium citrate preparation is used for the management of renal tubular acidosis (RTA) with calcium stones (nephrolithiasis); calcium oxalate stones by any cause, and uric acid nephrolithiasis (with or without calcium stones). This regimen also includes adequate water intake (leading to a urine out put of 2 L/day or more) and sodium restriction.
Definition
potassium: Symbol K. A soft silverymetallic element belonging to group1 (formerly IA) of the periodic table(see alkali metals); a.n. 19; r.a.m.39.098; r.d. 0.86; m.p. 63.7°C; b.p.774°C. The element occurs in seawaterand in a number of minerals,such as sylvite (KCl), carnallite(KCl·MgCl2·6H2O), and kainite(MgSO4·KCl·3H2O). It is obtained byelectrolysis. The metal has few usesbut potassium salts are used for awide range of applications. Potassiumis an essential element for livingorganisms. The potassium ion,K+, is the most abundant cation inplant tissues, being absorbed throughthe roots and being used in suchprocesses as protein synthesis. In animalsthe passage of potassium andsodium ions across the nerve-cellmembrane is responsible for thechanges of electrical potential thataccompany the transmission of impulses.Chemically, it is highly reactive,resembling sodium in itsbehaviour and compounds. It alsoforms an orange-coloured superoxide,KO2, which contains the O2- ion.Potassium was discovered by SirHumphry Davy in 1807.
Production Methods
Potassium superoxide (KO2) can create oxygen from water vapor (H2O) and carbon dioxide (CO2) and is used in respiratory equipment and is produced by burning potassium metal in dry air.
Preparation
Potassium metal is not produced commercially by a fused salt electrolysis of the chloride
—as is sodium—for several reasons: the metal is too soluble in the molten chloride to
separate and float on top of the bath; potassium metal vapors may also issue from the
molten bath, thus creating hazardous conditions; and potassium superoxide may form in the
cell and react explosively with potassium metal. Consequently, the established method of
preparing potassium metal commercially? involves the reduction of molten potassium
chloride by metallic sodium at elevated temperatures (850°C). Molten potassium chloride
is fed into the midpoint of a steel vessel provided with a fractionating tower packed with
stainless steel rings. Sodium is vaporized at the bottom and rises countercurrent to the
molten potassium chloride with which it reacts according to the equilibrium expression.
Although the left-hand side of the equation is favored thermodynamically, the escape of the
potassium vapors causes the reaction to proceed very efficiently to the right. The potassium
vapors are condensed and the product normally contains sodium metal as the only major
impurity up to about 1 % by weight. This product is sometimes purified by fractionating it in
a 38 ft high 316 stainless steel tower equipped with a reflux return reservoir. The condensate
is potassium metal of 99.99 % purity.
General Description
Potassium is potassium mixed with some other metal, usually sodium. Potassium is a liquid under normal conditions. Potassium reacts vigorously with water to form potassium hydroxide, a corrosive material and hydrogen, a flammable gas. The heat from this reaction may be sufficient to ignite the hydrogen. Potassium alloy may ignite spontaneously in contact with air. Once ignited, potassium burns quite violently. Potassium is used as a heat exchange fluid.
Air & Water Reactions
Reacts vigorously with oxygen. Reacts vigorously with water even at less than 100°C [Merck, 11th ed., 1989]. Water (caustic solution, H2) The oxidation of potassium in air is so rapid that the heat generated by the reaction melts and ignites the metal. This is particularly the case when pressure is applied at ordinary temperatures [Sidgwick 1. 1950]. Potassium burns in moist air at room temperature [Mellor 2:468. 1946-47]. The higher oxides of potassium, formed in air, react explosively with pure potassium, sodium, sodium-potassium alloys, and organic matter [Mellor 2, Supp. 3:1559. 1963].
Reactivity Profile
Boron trifluoride reacts with incandescence when heated with alkali metals or alkaline earth metals except magnesium [Merck 11th ed. 1989]. Maleic anhydride decomposes explosively in the presence of alkali metals . Sodium peroxide oxidizes antimony, arsenic, copper, potassium, tin, and zinc with incandescence . Alkali metal hydroxides, acids, anhydrous chlorides of iron, tin, and aluminum, pure oxides of iron and aluminum, and metallic potassium are some of the catalysts that may cause ethylene oxide to rearrange and polymerize, liberating heat . Explosions occur, although infrequently, from the combination of ethylene oxide and alcohols or mercaptans [Chem. Eng. News 20:1318. 1942]. A mixture of potassium and any of the following metallic halides produces a strong explosion on impact: aluminum chloride, aluminum fluoride, ammonium fluorocuprate, antimony tribromide, antimony trichloride, antimony triiodide, cadmium bromide, cadmium chloride, cadmium iodide, chromium tetrachloride, cupric bromide, cupric chloride, cuprous bromide cuprous chloride, cuprous iodide, manganese chloride, mercuric bromide, mercuric chloride, mercuric fluoride, mercuric iodide, mercurous chloride, nickel bromide, nickel chloride, nickel iodide, silicon tetrachloride, silver fluoride, stannic chloride, stannic iodide (with silver), stannous chloride, sulfur dibromide, thallous bromide, vanadium pentachloride, zinc bromide, zinc chloride, and zinc iodide [Mellor 2, Supp. 3:1571. 1963]. A mixture of potassium and any of the following compounds produces a weak explosion on impact: ammonium bromide, ammonium iodide, cadmium fluoride, chromium trifluoride, manganous bromide, manganous iodide, nickel fluoride, potassium chlorocuprate, silver chloride, silver iodide, strontium iodide, thallous chloride, and zinc fluoride [Mellor 2, Supp. 3:1571. 1963]. A mixture of potassium and any of the following compounds may explode on impact: boric acid, copper oxychloride, lead oxychloride, lead peroxide, lead sulfate, silver iodate, sodium iodate, and vanadium oxychloride [Mellor 2, Supp. 3:1571. 1963]. A mixture of potassium with any of the following compounds produces a very violent explosion on impact: boron tribromide, carbon tetrachloride, cobaltous bromide, cobaltous chloride, ferric bromide, ferric chloride, ferrous bromide, ferrous chloride, ferrous iodide, phosphorus pentachloride, phosphorus tribromide, and sulfur dichloride [Mellor 2, Supp. 3:1571. 1963]. Mixture of solid potassium and carbon dioxide(as dry ice) explodes when subjected to shock [Mellor 2, Supp. 3:1568. 1963]. Potassium and its alloys form explosive mixtures with chlorinated hydrocarbons [Chem. Eng. News 26:2604. 1948]. Ethylene oxide is dangerously reactive with metallic potassium [Chemical Safety Data Sheet SD-38:11. 1951]. Potassium in contact with the following oxides causes an explosive reaction: potassium ozonide, potassium peroxide, or potassium superoxide [Mellor 2, Supp. 3:1577. 1963].
Hazard
Elemental potassium as a metal is not found in its pure form in nature, but is derived fromits numerous compounds. The metal is very dangerous to handle. It can ignite while you areholding it with your hands or as you cut it. The metal must be stored in an inert gas atmosphereor in oil. Potassium fires cannot be extinguished with water—it only makes matters worsebecause it results in the formation of potassium hydroxide and hydrogen gas with enough heatto ignite the hydrogen. Dry chemicals such as soda ash, graphite, or dry sand can be used.
A particular hazard, which has been with humans since the beginning of time, is theradioactive isotope potassium-40 (K-40). Less than 1% of all potassium atoms on Earth arein the form of this radioactive isotope. It has a half-life of 1.25 billion years. Its decay process ends with the formation of the noble gas argon, which can then be analyzed to determine theage of rocks. This system (K-40 → argon) has been used to establish that the oldest rocks onEarth were formed about 3.8 billion years ago. Every living thing needs some potassium inits diet, including humans, who cannot escape this source of radiation, given that the humanbody cannot distinguish the radioactive potassium from the nonradioactive form. Along withcosmic rays and other naturally radioactive elements in the Earth’s crust, potassium-40 contributesto the normal lifetime accumulation of radiation. It makes up almost one-fourth ofthe total radiation the human body receives during a normal life span.
Health Hazard
Potassium reacts with the moisture on skin and other tissues to form highly corrosive potassium hydroxide. Contact of metallic potassium with the skin, eyes, or mucous membranes causes severe burns; thermal burns may also occur due to ignition of the metal and liberated hydrogen.
Flammability and Explosibility
Potassium metal may ignite spontaneously on contact with air at room temperature. Potassium reacts explosively with water to form potassium hydroxide; the heat liberated generally ignites the hydrogen formed and can initiate the combustion of potassium metal itself. Potassium fires must be extinguished with a class D dry chemical extinguisher or by the use of sand, ground limestone, dry clay or graphite, or "Met-L-X?" type solids. Water or CO2, extinguishers must never be used on potassium fires.
Agricultural Uses
Since the beginning of the 19th century, potassium has
been recognized as an essential element and a major
nutrient for plant growth, needed in large quantities. The
exact function of potassium is not fully understood.
Potassium makes plants more resistant to fimgal
diseases and insect attacks. It is good for healthy root
development and crop quality. For instance, potassium
improves the (a) texture, color and combustibility of
tobacco leaf, (b) sugar, starch and oil content in many
plants, and (c) taste, size and keeping quality of fruits.
Potato, tobacco and sugar use potassium, especially
during their early growth stages. A small quantity of
potassium is essential near young seedlings, while an
excessive quantity causes salt damage.
The requirement of potassium varies in growing
plants. Most seeds contain 0.1 to 10% potassium, which
is sufficient for germination and early growth. The
vegetative growth is characterized by a progressive
increase in the absorption of inorganic elements like
potassium. In tobacco, potassium is absorbed at the rate
of 0.1 kglhalday from the 2lst day of transplanting; a
maximum uptake of 2 kg/ha/day occurs 49 days after
transplanting. The minimum level of readily available
potassium in the soil is around 175 kg/ha.
Potassium is present in the cell sap solution or
plasma, and is almost fully extractable with water from
plant tissues. It accumulates at the site of cell division,
and helps in maintaining the physiological state of the
swelling of plasma colloids which is necessary for all
normal metabolic processes. It maintains the balance of
anabolism, respiration and transpiration of a plant or
leaf, and keeps the plant's water economy in equilibrium
(in turn, reducing the plant's tendency to wilt.)
Potassium has a very important role to play in plant
energy metabolism. Its liberal use helps to harden the
supporting tissues which, in turn, improves the keeping
qualities of fruits, and consequently leads to a stronger
structure.
Potassium does not become a part of the plant
structure as P, S, Ca and Mg do. But it helps in carbon
dioxide assimilation, translocation of proteins and
sugars, enzyme activity, cell division, reduction of
nitrates and fat synthesis. The influence of potassium in
these activities is now well established.
The levels of potassium and nitrogen are closely
related in most plants. Nitrogen stimulates the rapid growth of soft tissues, whereas potassium promotes the
growth of soft tissues. If sufficient potassium is
unavailable, nitrogen level increases in the outer leaves
of cabbage and in the upper stems and leaves of tomato.
In the sheath tissue of sugar cane, the relationship of
potassium to nitrogen depends on their respective
concentrations.
Ammonium has a greater depressing effect on
potassium in soil-grown plants than in solution, because
ammonium interferes with the diffusion of potassium
from the clay lattice. Potassium influences the uptake of
the two forms of nitrogen. The relative presence of K, Ca
and Mg influences the concentration of each individual
cation within the plant. In this, potassium seems to be the
most active. In plants, magnesium has a greater
depressing effect on the content of potassium than that of
calcium.
Because potassium ions (K+)an d sodium ions (Na+)
are similar in size and chemical properties, sodium may
replace potassium in several essential roles. However,
potassium is an essential element, whereas sodium is not.
Therefore, use of sodium may compensate for the
potassium shortage to some extent, but sodium will not
produce healthy plants in a situation when potassium
deficiency is large.
There is a close relationship between carbohydrates
and the potassium level. When soil potassium
concentration is insufficient for optimum growth, it is
commonly transported from more mature tissues to the
meristems, so that older leaves exhibit early deficiency
symptoms. Chlorosis appears first around the edges and
tips of the leaves, and then spreads to the mid rib,
followed finally by necrosis.
In many crops, potassium deficiency is characterized
by a contrast between chlorosis, necrosis and healthy
green areas of leaves. In the advanced stages of
potassium starvation in corn, leaf edges become necrotic,
the tissue disintegrates, and the leaf gets a ragged
appearance. This condition is called leaf scorch.
Potassium deficiency in alfalfa is seen as white spots on
the leaf edges, whereas chlorosis and necrosis of leaf
edges are observed in other grasses.
Potassium deficiency can also occur among young
upper leaves in some high-yielding , fast-maturing crops
like cotton and wheat. Insufficient potassium weakens the
straw in grain crops, causes lodging in small grains and
stalk breakage in corn and sorghum. Potassium
deficiencies greatly reduce crop yield. A phenomenon in
which deficiency symptoms are not visible is called
hidden hunger. Potassium stress increases the degree of
crop damage by bacterial and fungal diseases, insect and
mite infestation, and nematode and virus infection. Lack
of potassium in wetland rice greatly increases the
sensitivity of foliar diseases such as stem rot, sheath
blight and brown leaf spot.
Soil humus is a major source of sulphur, but not of
potassium. Potassium ion is a highly soluble cation in
solution, but it moves slowly in soils (unlike sulphur
which is soluble and a readily mobile sulphate ion).
Diffusion and mass flow of potassium to plant roots
account for a large portion of absorbed potassium. In
decaying humus, the potassium ion is fust leached into
the soil solution and then to cation exchange sites on the
humus and clay particles. A non-decomposed organic
mass added to the soil replaces large amounts of
potassium which flows with the water to the roots.
In plant cells, potassium is the most abundant metal
cation. On decomposition, fresh plant residues give all
the potassium the plant needs for growth as a mobile
soluble ion. Soluble potassium can be immobilized into
the bodies of microbes, lost in leaching waters, or held
between layers of hydrous mica and similar clays during
drying. High yielding crop plants take potassium ions
from a small reservoir of readily available potassium,
namely the exchangeable source. For a good crop, at
least 170 to 200 kg/ha potassium is considered essential.
Soluble potassium may suffice if the soil is neutral or
basic.
Using potassium fertilizers in excess, or too
frequently, may result in an excess uptake of potassium
by plants and in lowering their potassium-magnesium
absorption. The effectiveness of the soil solutionpotassium
for crop uptake is influenced by the presence
of other cations, especially Na, Ca, Mg and Al. The
absorption of potassium, in excess of that required for
optimum growth, results in the accumulation of the
nutrient without a corresponding increase in the growth,
and is known as luxury consumption. The exchangeable
or water-soluble potassium is converted by the
potassium furation process to a form, not easily
exchangeable from the adsorption complex, by a cation
of a neutral salt solution.
Pharmacokinetics
Potassium maintains an electrolyte gradient on cell surfaces, keeping at specific concentrations inside and outside of the cell; this impacts fluid and electrolyte balance, nerve transmission, muscle contraction, as well as cardiac and kidney function. Clinical evidence has associated potassium intake with lower blood pressure in adults, reducing the risk stroke and heart disease. Dietary potassium may exert beneficial effects on bone loss in the elderly and kidney stones. Consumption of white vegetables, which are normally high in potassium, is associated with a lower risk of stroke.
A note on gastrointestinal lesions
Potassium in solid oral preparations (for example, tablets) can cause ulcerative or stenotic lesions in the esophagus and stomach. Use diluted liquid potassium preparations or injection preparations if there are concerns about gastrointestinal health.
Safety Profile
The toxicity of potassium compounds is almost always that of the anion, not of potassium. A dangerous fire hazard. Metallic potassium reacts with moisture to form potassium hydroxide and hydrogen. The reaction evolves much heat, causing the potassium to melt and spatter. The reaction also ignites the hydrogen, which burns, or if there is any confinement, may explode. It can ignite spontaneously in moist air. Store under mineral oil. Potassium metal wdl form the peroxide (K2O2) and the superoxide (KO3 or K2O4) at room temperature even when stored under mineral oil. These oxides can explode on contact with organic materials. Metal that has oxidized on storage under oil may explode violently when handled or cut. Oxide-coated potassium should be destroyed by burning. Danger: burning potassium is difficult to extinguish; dry powdered soda ash or graphte or special mixtures of dry chemical are recommended. A violent explosion hazard with the following materials under required conditions of temperature, pressure, and state of division: acetylene, air, moist air, alcohols (e.g., n-propanol through n-octanol, benzyl alcohol, cyclohexanol), AlBr3, ammonium nitrate + ammonium sulfate, ammonium chlorocuprate, NHdi, NH41, antimony halides, arsenic hahdes, AsH3 + NH3, Bi203, boric acid, BBr3, carbon disulfide (impact-sensitive), solid carbon dioxide, carbon monoxide, chlorinated hydrocarbons (e.g., chloroethane, dichloroethane, dchloromethane, trichloroethane, chloroform, pentachloro- ethane, carbon tetrachloride, tetrachloro- ethane), halocarbons (e.g., bromoform, dbromomethane, diiodomethane) , iodme (impact-sensitive), interhalogens (e.g., chlorine trifluoride, iodine bromide, iodine chloride, iodine pentafluoride, iodme trichloride), ClO, CrO3, Cu2OCl2, CuO, ethylene oxide, fluorine, graphite, graphte + air, graphite + K2O2, hydrogen iodide, H2O2, hydrogen chloride, hydrazine, Pb2OCl2, PbO2, PbSO4, maleic anhydride, metal halides (e.g., calcium bromide, iron(Ⅲ) bromide, iron(Ⅲ) chloride, iron(Ⅱ) chloride, iron(Ⅱ) bromide, iron(Ⅱ) iodide, cobalt(Ⅱ) chloride, chromium tetrachloride, silver fluoride, mercury(Ⅱ) bromide, mercury(Ⅱ) chloride, mercury(Ⅱ) fluoride, mercury(Ⅱ) iodide, copper0 chloride,copper(Ⅰ) iodde, copper(Ⅱ) bromide, copper(Ⅱ) chloride, ammonium tetrachlorocuprate, zinc chlorides, bromides, or ioddes, cadmium chlorides, bromides or iodides, aluminum fluorides, chlorides, or bromides, thalliump) bromide, tin chlorides, tin iodide, arsenic trichloride, arsenic triiodde, antimony tribromides, trichlorides or triiodides, bismuth tribromides, trichlorides, or triioddes, vanadiumo chloride, manganese(Ⅰ) chloride, nickel bromide, chloride, or iodide), metal oxides (e.g., lead peroxide, mercury(Ⅰ) oxide, MoO3, nitric acid, nitrogen-containing explosives (e.g., ammonium nitrate, picric acid, nitrobenzene), nonmetal halides (e.g., diselenium dichloride, seleninyl chloride, seleninyl bromide, sulfur dichloride, sulfur dibromide, phosphorus tribromide, phosphorus trichloride, phosgene, disulfur dichloride), nonmetal oxides (e.g., dichlorine oxide, dinitrogen tetraoxide, dinitrogen pentaoxide, NO2, P2O5), oxalyl dibromide, oxalyl dichloride, P2NF, peroxides, COCl2, PH3 + NH3, phosphorus, PCl5, PBr3, potassium chlorocuprate, potassium oxides (e.g., KO3, K2O2, KO2), selenium, SeOCl2, SiCl4, AglO3, NalO3, NH3 + NaNO2, Na2O2, SnI4 + S, SnO2, S, sulfuric acid, tellurium, thiophosphoryl fluoride, VOCl2, water. Other hazardous reactions may occur with carbon (e.g., soot, graphte, activated charcoal), dimethyl sulfoxide, ethylene oxide, chlorine, bromine vapor, hydrogen bromide, potassium iodide + magnesium bromide, chloride or iodide, maleic anhydride, mercury, copper(Ⅱ) oxide, mercury(Ⅱ) oxide, tin(Ⅳ) oxide, molybdenum(Ⅲ) oxide, bismuth trioxide, phosphorus trichloride, sulfur dioxide, chromium trioxide. toxic fumes of K2O. When heated to decomposition it emits
Potential Exposure
Used as a reagent and in sodiumpotassium alloys which are used as high-temperature heat transfer media.
Environmental Fate
Potassium metal in the environment will react with air, oxidizing the exposed surfaces, and reacts violently with water, yielding potassium hydroxide and hydrogen gas, which reacts with oxygen in air, producing flame.
Metabolism
Potassium is absorbed and excreted in unchanged form.
Storage
Safety glasses, impermeable gloves, and a fire-retardant laboratory coat should be worn at all times when working with potassium, and the metal should be handled under the surface of an inert liquid such as mineral oil, xylene, or toluene. Potassium should be used only in areas free of ignition sources and should be stored under mineral oil in tightly sealed metal containers under an inert gas such as argon. Potassium metal that has formed a yellow oxide coating should be disposed of immediately; do not attempt to cut such samples with a knife since the oxide coating may be explosive.
Shipping
UN2257Potassium, Hazard Class: 4.3; Labels: 4.3-Dangerous when wet material. UN1420 Potassium, metal alloys and metal alloys, liquid, Hazard Class: 4.3; Labels: 4.3-Dangerous when wet material. UN3089 Metal powder, flammable, n.o.s. Hazard Class: 4.2; Labels: 4.2-Spontaneously combustible material.
Toxicity evaluation
Potassium is a cofactor and activates a large variety of enzymes, including glycerol dehydrogenase, pyruvate kinase, L-threonine dehydrase, and ATPase. Its acute toxicity is primarily due to its action as an electrolyte. Excessive or diminished potassium levels can disrupt membrane excitability and influence muscle cell contractility and neuronal excitability.
Incompatibilities
Air contact causes spontaneous ignition. Violent reaction with water, forming heat, spattering, corrosive potassium hydroxide and explosive hydrogen. The heat from the reaction can ignite the hydrogen that is generated. A powerful reducing agent. Violent reaction with oxidizers, organic materials; carbon dioxide; heavy metal compounds; carbon tetrachloride; halogenated hydrocarbons; easily oxidized materials; and many other substances. Store under nitrogen, mineral oil, or kerosene. Oxidizes and forms unstable peroxides under storage conditions. Potassium metal containing an oxide coating is an extremely dangerous explosion hazard and should be removed by an expert and destroyed.
Waste Disposal
Excess potassium and waste material containing this substance should be placed in an appropriate container under an inert atmosphere, clearly labeled, and handled according to your institution's waste disposal guidelines. Experienced personnel can destroy small scraps of potassium by carefully adding t-butanol or nbutanol to a beaker containing the metal scraps covered in an inert solvent such as xylene or toluene.
Properties of Potassium
| Melting point: | 64 °C (lit.) |
| Boiling point: | 760 °C (lit.) |
| Density | 0.86 g/mL at 25 °C (lit.) |
| vapor pressure | 0.09 mm Hg ( 260 °C) |
| refractive index | n |
| storage temp. | 2-8°C |
| solubility | H2O: soluble |
| form | rod |
| color | Silver/gray |
| Specific Gravity | 0.86 |
| Odor | Odorless |
| PH | 5.0 (H2O, 20°C) |
| Resistivity | 6.1 μΩ-cm, 20°C |
| Water Solubility | reacts |
| Sensitive | Air & Moisture Sensitive |
| Exposure limits | ACGIH: TWA 2 ppm; STEL 4 ppm OSHA: TWA 2 ppm(5 mg/m3) NIOSH: IDLH 25 ppm; TWA 2 ppm(5 mg/m3); STEL 4 ppm(10 mg/m3) |
| Dielectric constant | 5(0.0℃) |
| Stability: | Stable. Moisture and air-sensitive. Spontaneously combustible through the generation and ignition of hydrogen. Reacts violently with water and acids, alcohols, carbon monoxide. Store under oil. |
| CAS DataBase Reference | 7440-09-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Potassium(7440-09-7) |
| EPA Substance Registry System | Potassium (7440-09-7) |
Safety information for Potassium
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H260:Substances And Mixtures Which, In Contact With Water,Emit Flammable Gases H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P223:Keep away from any possible contact with water, because of violent reaction and possible flash fire. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P231+P232:Handle under inert gas. Protect from moisture. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Potassium
Potassium manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

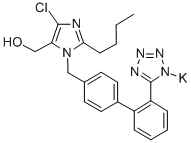
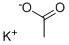
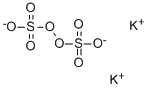
You may like
-
 Potassium CAS 7440-09-7View Details
Potassium CAS 7440-09-7View Details
7440-09-7 -
 Potassium CASView Details
Potassium CASView Details -
 Potassium metal CAS 7440-09-7View Details
Potassium metal CAS 7440-09-7View Details
7440-09-7 -
 Conductivity Standard CASView Details
Conductivity Standard CASView Details -
 Potassium standard CASView Details
Potassium standard CASView Details -
 Conductivity Standard CASView Details
Conductivity Standard CASView Details -
 Conductivity Standard CASView Details
Conductivity Standard CASView Details -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
