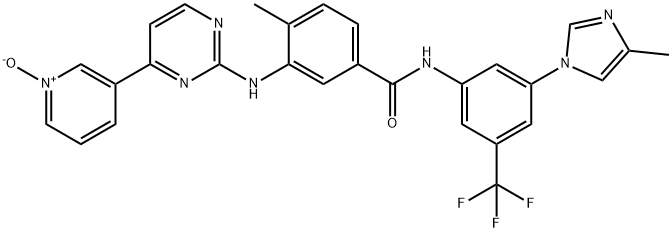
Nilotinib
- CAS NO.:641571-10-0
- Empirical Formula: C28H22F3N7O
- Molecular Weight: 529.52
- MDL number: MFCD09833716
- EINECS: 700-544-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18
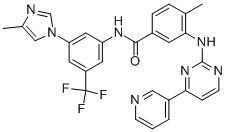
What is Nilotinib?
Absorption
Orally available
Description
Chronic myeloid leukemia (CML), a hematological stem-cell disorder, is definitively diagnosed by the detection of the Philadelphia chromosome, a truncated version of chromosome 22 resulting from the reciprocal translocation of chromosomes 9 and 22 induced by a single mutagenic event. The consequence is the juxtaposition of two genes creating a fusion gene BCR-ABL. This gene leads to the translation of a fusion protein with increased tyrosine kinase activity that contributes to the pathogenesis of CML. Targeting the BCR-ABL protein has led to the successful intervention of the disease. Now established as first-line therapy for CML, imatinib was the first selective tyrosine kinase inhibitor of BCR-ABL. Since imatinib only binds to an inactive conformation of the ABL kinase portion, the conformational restrictions contribute to its selectivity.
Chemical properties
Off-White Solid
Originator
Novartis (Switzerland)
The Uses of Nilotinib
Nilotinib, an orally active signal transduction inhibitor that selectively inhibits the tyrosine kinase Bcr-Abl, was discovered and developed by Norvartis and was launched for the treatment of chronic myeloid leukemia (CML) in patients with Philadelphia chromosome-positive (Ph+) disease who are resistant or intolerant to imatinib mesilate. Additional clinical trials are currently underway for the treatment of acute lymphoblastic leukemia (ALL) and gastrointestinal stromal tumors (GISTs).
The Uses of Nilotinib
Nilotinib (AMN-107) is a Bcr-Abl inhibitor with IC50 less than 30 nM.
The Uses of Nilotinib
Nilotinib-d6, is the labeled analogue of Nilotinib, which might be useful in treatment of chronic myelogenous leukemia.
Background
Nilotinib, also known as AMN107, is a tyrosine kinase inhibitor under investigation as a possible treatment for chronic myelogenous leukemia (CML). A Phase I clinical trial in 2006 showed that this drug was relatively safe and offered significant therapeutic benefits in cases of CML which were found to be resistant to treatment with imatinib (Gleevec), another tyrosine kinase inhibitor used as a first-line treatment for CML.
Indications
For the potential treatment of various leukemias, including chronic myeloid leukemia (CML).
What are the applications of Application
Nilotinib is a rationally designed c-Abl and Bcr-Abl kinase inhibitor
Definition
ChEBI: Nilotinib is a member of (trifluoromethyl)benzenes, a member of pyrimidines, a member of pyridines, a member of imidazoles, a secondary amino compound and a secondary carboxamide. It has a role as an antineoplastic agent, a tyrosine kinase inhibitor and an anticoronaviral agent.
brand name
Tasigna
General Description
Class: non-receptor tyrosine kinase
Treatment: CML
Elimination half-life = 17 h
Protein binding >97.5%
Pharmacokinetics
Nilotinib is a transduction inhibitor that targets BCR-ABL, c-kit and PDGF, for the potential treatment of various leukemias, including chronic myeloid leukemia (CML).
Clinical Use
Tyrosine kinase inhibitor:
Treatment of chronic myelogenous leukaemia
(CML)
Synthesis
The first step in the synthesis of nilotinib involves the nucleophilic aromatic substitution of 3-fluoro-5-(trifluoromethyl)benzonitrile with 2-methylimidazole. The nitrile is then hydrolyzed with sodium hydroxide in aqueous dioxane. A Curtius rearrangement employing diphenylphosphoryl azide in tert-butanol affords the tert-butyl carbamate. Deprotection of the Boc group provides the 3-(4-methylimidazol-1-yl)-5-(trifluoromethyl)aniline piece for the convergent synthesis. Construction of the other half begins with the condensation of 3-amino-4- methylbenzoic acid methyl ester with cyanamide in refluxing ethanolic HCl to generate the 3-guanidinobenzoate. An enamino ketone, prepared by a Claisen condensation of 3-acetylpyridine with ethyl formate in the presence of sodium metal in hot toluene, is then cyclized with the guanidine to yield the pyridylpyrimidine. Following saponification of the ethyl ester, the resultant 4-methyl-3-[4-(3-pyridyl)pyrimidin-2-ylamino]benzoic acid is finally coupled with the aniline utilizing diethyl cyanophosphate to provide nilotinib.
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: avoid with clarithromycin, rifampicin
(concentration reduced) and telithromycin.
Antifungals: avoid with itraconazole, ketoconazole
(concentration increased) and voriconazole.
Antipsychotics: avoid with clozapine (increased risk
of agranulocytosis).
Antivirals: avoid with boceprevir and ritonavir
(concentration possibly increased).
Grapefruit juice: avoid concomitant administration.
Avoid concomitant use with other inhibitors or
inducers of CYP3A4. Dose alterations may be
required.
Metabolism
Not Available
Metabolism
Nilotinib is metabolised in the liver via oxidation and hydroxylation, in which cytochrome P450 isoenzyme CYP3A4 plays an important role. Most of an oral dose is eliminated unchanged in the faeces within 7 days.
References
1) Weisberg?et al.?(2006),?AMN107 (nilotinib): a novel and selective inhibitor of BCR-ABL; Br J. Cancer,?94?1765 2) Verstovsek?et al.?(2006),?Activity of AMN107, a novel aminopyrimidine tyrosine kinase inhibitor, against human FIP1L1-PDGFR-alpha-expressing cells; Ann. Neurol.,?75?209
Properties of Nilotinib
| Melting point: | 231-233 °C |
| Density | 1.36 |
| storage temp. | -20°C Freezer |
| solubility | Soluble in DMSO (up to 50 mg/ml) |
| form | Beige powder. |
| color | Off-white |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 1 month. |
| CAS DataBase Reference | 641571-10-0(CAS DataBase Reference) |
Safety information for Nilotinib
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Nilotinib
| InChIKey | HHZIURLSWUIHRB-UHFFFAOYSA-N |
| SMILES | N1(C=C(C)N=C1)C1C=C(C=C(C=1)NC(=O)C1C=CC(C)=C(NC2=NC=CC(C3=CC=CN=C3)=N2)C=1)C(F)(F)F |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
![641571-10-0 4-Methyl-N-[3-(4-Methyl-1H-Imidazol-1-yl)-5-(Trifluoromethyl) Phenyl]-3-[[4-(3-Pyridinyl)-2-Pyrimidinyl] Amino] Benzamide 98%](https://img.chemicalbook.in//ProductImageIndia/2024-03/Raw/4cb18bb8-250c-4b01-ad4b-f37c8770a175.png) 641571-10-0 4-Methyl-N-[3-(4-Methyl-1H-Imidazol-1-yl)-5-(Trifluoromethyl) Phenyl]-3-[[4-(3-Pyridinyl)-2-Pyrimidinyl] Amino] Benzamide 98%View Details
641571-10-0 4-Methyl-N-[3-(4-Methyl-1H-Imidazol-1-yl)-5-(Trifluoromethyl) Phenyl]-3-[[4-(3-Pyridinyl)-2-Pyrimidinyl] Amino] Benzamide 98%View Details
641571-10-0 -
 Nilotinib 641571-10-0 98%View Details
Nilotinib 641571-10-0 98%View Details
641571-10-0 -
 NILOTINIB 95-99 %View Details
NILOTINIB 95-99 %View Details -
 Nilotinib 98% CAS 641571-10-0View Details
Nilotinib 98% CAS 641571-10-0View Details
641571-10-0 -
 Nilotinib 98% (HPLC) CAS 641571-10-0View Details
Nilotinib 98% (HPLC) CAS 641571-10-0View Details
641571-10-0 -
 Nilotinib for system suitability CAS 641571-10-0View Details
Nilotinib for system suitability CAS 641571-10-0View Details
641571-10-0 -
 Nilotinib CAS 641571-10-0View Details
Nilotinib CAS 641571-10-0View Details
641571-10-0 -
 Ninlib hetero nilotinib 150mg Capsules CML, For Personal, Packaging Size: 7*4 CapsuleView Details
Ninlib hetero nilotinib 150mg Capsules CML, For Personal, Packaging Size: 7*4 CapsuleView Details
641571-10-0
