Phosphorus
Synonym(s):P;Phosphorus;CAF;PCAF;Phosphorene
- CAS NO.:7723-14-0
- Empirical Formula: P
- Molecular Weight: 30.97
- MDL number: MFCD00133771
- EINECS: 231-768-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:40
What is Phosphorus?
Description
White or yellow white phosphorus is a yellow waxy or colourless, transparent, volatile crystalline solid, waxy appearance with a garlic-like odour. On exposure to light, it darkens and ignites in air. It is also called yellow phosphorus colour because of impurities. White phosphorus does not occur naturally but is manufactured from phosphate rocks. It is insoluble in water, slightly soluble in benzene, ethanol, and chloroform, and is soluble in carbon disulphide. White phosphorus reacts rapidly with oxygen, easily catching fire at temperatures 10°C–15°C above room temperature. White phosphorus is used by the military in various types of ammunition and to produce smoke for concealing troop movements and identifying targets. It is also used by industry to produce phosphoric acid and other chemicals for use in fertilisers, food additives, and cleaning compounds. Small amounts of white phosphorus were used in the past in pesticides and fireworks.White phosphorus is used mainly for producing phosphoric acid and other chemicals. These chemicals are used to make fertilisers, additives in foods and drinks, cleaning compounds, and other products. In the military, white phosphorus is used in ammunitions such as mortar and artillery shells, and grenades.
Chemical properties
reddish-brown powder
Chemical properties
Yellow or white phosphorus ignites spontaneously in air at 34 °C. It should be stored under water. Under this condition, however, it may form phosphoric acid. Stainless steel containers should be used to hold the corrosive material. White phosphorus fires can be controlled by using water or sand or by excluding air.
Physical properties
Although phosphorus is in group 15 with some other metalloids, it is usually classed as anonmetal since it resembles nitrogen somewhat, the element above it in group 15. Both areessential to the biochemical field as vital elements to support life. Phosphorus has 10 knownallotropic forms. This is an unusually high number for any element. A system of categorizingthe allotropes by three colors has made it easier to keep track of them. These three colors arewhite, red, and black phosphorus.
White phosphorus has a white waxy appearance that turns slightly yellow with age andimpurities. There are two allotropic forms of white phosphorus. The alpha (α) form has acubic crystal structure, and the beta (β) form has a hexagonal crystalline structure. Whitephosphorus is extremely reactive and will spontaneously burst into flame when exposed to airat a temperature of about 35°C. It must be kept under water. But this property of spontaneouscombustion has made it useful for military applications.
White phosphorus is the most useful version of the three allotropes, and it is used inprocesses to manufacture the other two versions of phosphorus. White phosphorus’s meltingpoint 44.15°C, its boiling point is 280.5°C, and its density is 1.82 c/cm3.
Exposing white phosphorus to a process of heat produces red phosphorus. Red phosphorushas a density of 2.34 g/cm3.
Black phosphorus also starts with heating white phosphorus. The difference is that thewhite phosphorus is heated in the presence of a mercury catalyst and a small amount ofalready-formed black phosphorus. Its density is 2.4 g/cm3.
Isotopes
There are a 23 isotopes of phosphorus, ranging from P-24 to P-46, with halflivesthat range from a few nanoseconds to about two and half minutes. The one stableisotope is phosphorus-31, which accounts for 100% of the natural phosphorus on Earth.
Origin of Name
Its name is derived from the Greek word phosphoros, which means “bringer of light” or “light bearing.”
Occurrence
Phosphorus is the 12th most abundant element. It makes up about 0.1% of the Earth’s crust.Phosphorous occurs in nature in several forms, mostly as phosphates. The most commonsource is phosphate rock [Ca3(PO4)2] and a mineral called “apatite.” Phosphorus is found inall animal bones and teeth and in most living tissue. Phosphorous nodules are found on theocean floor along with manganese nodules.Most commercial phosphorus is produced in electric furnaces where the phosphate-richminerals are heated to drive off the phosphorus as a gas, which is then condensed under water.Another process uses sulfuric acid to remove the phosphorus.
History
Discovered in 1669 by Brand, who prepared it from urine. Phosphorus exists in four or more allotropic forms: white (or yellow), red, and black (or violet). White phosphorus has two modifications: α and β with a transition temperature at –3.8°C. Never found free in nature, it is widely distributed in combination with minerals. Twenty-one isotopes of phosphorus are recognized. Phosphate rock, which contains the mineral apatite, an impure tricalcium phosphate, is an important source of the element. Large deposits are found in the Russia, China, Morocco, and in Florida, Tennessee, Utah, Idaho, and elsewhere. Phosphorus in an essential ingredient of all cell protoplasm, nervous tissue, and bones. Ordinary phosphorus is a waxy white solid; when pure it is colorless and transparent. It is insoluble in water, but soluble in carbon disulfide. It takes fire spontaneously in air, burning to the pentoxide. It is very poisonous, 50 mg constituting an approximate fatal dose. Exposure to white phosphorus should not exceed 0.1 mg/m3 (8-hour time-weighted average — 40- hour work week). White phosphorus should be kept under water, as it is dangerously reactive in air, and it should be handled with forceps, as contact with the skin may cause severe burns. When exposed to sunlight or when heated in its own vapor to 250°C, it is converted to the red variety, which does not phosphoresce in air as does the white variety. This form does not ignite spontaneously and it is not as dangerous as white phosphorus. It should, however, be handled with care as it does convert to the white form at some temperatures and it emits highly toxic fumes of the oxides of phosphorus when heated. The red modification is fairly stable, sublimes with a vapor pressure of 1 atm at 417°C, and is used in the manufacture of safety matches, pyrotechnics, pesticides, incendiary shells, smoke bombs, tracer bullets, etc. White phosphorus may be made by several methods. By one process, tricalcium phosphate, the essential ingredient of phosphate rock, is heated in the presence of carbon and silica in an electric furnace or fuel-fired furnace. Elementary phosphorus is liberated as vapor and may be collected under water.
Characteristics
White phosphorus occurs in nature in phosphate rock. It is insoluble in water and alcoholand will ignite spontaneously in air. It exhibits what is known as phosphorescence; that is, itglows in the dark at room temperature. White phosphorus is poisonous and must be storedunder water.
Red phosphorus is less reactive than the white variety. It is not poisonous, but largeamounts can explode. It is used in fireworks and matches.
Black phosphorus is the only one of the three that will conduct electricity; white and redare poor conductors. Black phosphorus has no significant commercial uses.
The Uses of Phosphorus
It is used to make safety matches, incendiary shells,andsmokebombs;inpyrotechnics;and in the manufacture of fertilizers, pesticides, phosphoric acid, and phosphorus halides.
The Uses of Phosphorus
The allotropes and compounds of phosphorus have many important uses and are anessential commercial commodity. Phosphorus is essential to all living tissue, both plant andanimal. It is the main element in the compound adenosine triphosphate (ATP), the mainenergy source for living things.
Red phosphors are formed either by heating white phosphorus or by exposing white phosphorusto sunlight. It is quite different from the explosive white phosphorus. For instance,when scratched on a surface, the heads of safety matches made of red phosphorus convert backto white phosphorus and ignite due to the heat of the slight friction of the match on a roughsurface. Red phosphorus is also used in fireworks, smoke bombs, and pesticides and to makephosphoric acid, electroluminescent paints, and fertilizers.
Most elemental phosphorus is used to manufacture phosphoric acid, a solid that is usedto produce triple-phosphate fertilizers. Some soils require large amounts of phosphorus toproduce a viable crop.
Sodium tripolyphosphate is the main phosphate found in detergents. It acts as a watersoftener and counteracts the elements that are responsible for “hard water” while at the sametime making the detergent a more effective cleaner.
The Uses of Phosphorus
Phosphorus is an essential constituent of plants and animals, being present in deoxyribonucleic acid (DNA), bones, teeth and other components of high biological importance. Phosphorus does not occur in its elemental state in nature, as it readily oxidises and therefore is deposited as phosphate rock. The remaining elements of group 15 are mostly obtained from minerals, but can also be found in their elemental form in the earth’s crust.
Definition
phosphorus: Symbol P. A nonmetallicelement belonging togroup 15 (formerly VB) of the periodictable; a.n. 15; r.a.m. 30.9738; r.d.1.82 (white), 2.34 (red); m.p. 44.1°C(α-white); b.p. 280°C (α-white). It occursin various phosphate rocks,from which it is extracted by heatingwith carbon (coke) and silicon(IV)oxide in an electric furnace (1500°C).Calcium silicate and carbon monoxideare also produced. Phosphorushas a number of allotropic forms.The α-white form consists of P4 tetrahedra(there is also a β-white formstable below –77°C). If α-white phosphorusis dissolved in lead andheated at 500°C a violet form is obtained.Red phosphorus, which is acombination of violet and whitephosphorus, is obtained by heatingα-white phosphorus at 250°C with airexcluded. There is also a black allotrope,which has a graphite-likestructure, made by heating whitephosphorus at 300°C with a mercurycatalyst. The element is highly reactive.It forms metal phosphides andcovalently bonded phosphorus(III)and phosphorus(V) compounds. Phosphorusis an essential element forliving organisms. It is an importantconstituent of tissues (especiallybones and teeth) and of cells, beingrequired for the formation of nucleic acids and energy-carrying molecules(e.g. ATP) and also involved in variousmetabolic reactions. The elementwas discovered by Hennig Brand(c. 1630–92) in 1669.
Production Methods
Elemental phosphorous is produced as a by-product or
intermediate in the production of phosphate fertilizer. Environmental
contamination with phosphorus results from its
manufacture into phosphorus compounds and during the
transport and use of these compounds. In the manufacturing
process, phosphate rock containing the mineral apatite (tricalcium
phosphate) is heated, and elementary phosphorus is
liberated as a vapor. Phosphorus is used to manufacture
explosives, incendiaries, smoke bombs, chemicals, rodenticides,
phosphor bronze, and fertilizer. The use of phosphate
fertilizers results in increased level of nutrients in fresh water and is a major source of environmental pollution
problem.
Phosphorus exists in several allotropic forms: white (or
yellow), red, and black (or violet). The last is of no industrial
importance. Elemental yellow phosphorus extracted from
bone was used to make “strike anywhere” matches. In 1845,
the occupational disease “phossy jaw,” a jaw bone necrosis,
was recognized in workers who manufactured such matches.
A prohibitive tax imposed in 1912 on matches made from
yellow phosphorus led to the use of less toxic materials, red
phosphorus and phosphorus sesquisulfide. The United States
appears to have lagged behind European countries in that
signatories of the Berne Convention of 1906 agreed not to
manufacture or import matches made with yellow phosphorus.
Occasional injuries continued to result from using
yellow phosphorus to manufacture fireworks until 1926,
when an agreement was reached to discontinue using yellow
phosphorus for this purpose.
The world production of elemental phosphorus exceeds
1,000,000 metric ton. It is manufactured either in electric or
blast furnaces. Both depend on silica as a flux for the
calcium present in the phosphate rock. Almost all of the
phosphorus produced is converted into phosphoric acid or
other phosphorus compounds.
Red phosphorus does not ignite spontaneously but
may be ignited by friction, static electricity, heating, or
oxidizing agents. Handling it in an aqueous solution helps
prevent fires.
General Description
A white or yellow colored semi-liquid. Transported at high temperatures. Insoluble in water and denser than water. Contact may cause burns to skin, eyes, and mucous membranes. May be toxic by ingestion, inhalation and skin absorption. May ignite upon exposure to air. Used to make other chemicals.
Air & Water Reactions
When exposed to air emits a green light and gives off white fumes. Ignites at 30°C in moist air, higher temperatures are required for ignition in dry air [Merck 11th ed. 1989]. The reactivity of phosphorus with oxygen or air depends on the allotrope of phosphorus involved and the conditions of contact, white (yellow) phosphorus being by far more reactive. White phosphorus readily ignites in air if warmed, finely divided, or under conditions where the slow oxidative isotherm cannot be dissipated. Contact with finely divided charcoal or lampblack promotes ignition, probably by the absorbed oxygen. Contact with amalgamated aluminum also promotes ignition [Mellor 1940 and 1971].
Reactivity Profile
WHITE PHOSPHORUS reacts with air (fire, acidic solution); sulfur and oxidants (fire, explosion). Bromine trifluoride reacts similarly with arsenic, boron, bromine, iodine, phosphorus, and sulfur [Mellor 2:113. 1946-47]. Bromoazide explodes on contact with antimony, arsenic, phosphorus, silver foil, or sodium. Red phosphorus reacts in the cold with selenium oxychloride evolving light and heat; white phosphorus reacts explosively [Mellor 10:906. 1946-47]. When thorium is heated with phosphorus, they unite with incandescence [Svenska Akad. 1829. p. 1].
Hazard
Many of the compounds of phosphorus are extremely dangerous, both as fire hazardsand as deadly poisons to the nervous system of humans and animals. Some of the poisonouscompounds (PClx) can be absorbed by the skin as well as inhaled or ingested. Flushing withwater is the only way to stop the burning of white phosphorus on the skin, but water doesnot affect the combustion of some phosphorus compounds. Although red phosphorus is notas dangerous or poisonous as white phosphorus, merely applying some frictional heating willinduce the red allotrope to change back to the explosive white allotrope (the striking of a safetymatch is an example).
Some of the main types of poisonous gases used in warfare have a phosphorus base. Manycountries stockpile these gases, but, by agreement, the supplies are being reduced.
Health Hazard
Fire will produce irritating, corrosive and/or toxic gases. TOXIC; ingestion of substance or inhalation of decomposition products will cause severe injury or death. Contact with substance may cause severe burns to skin and eyes. Some effects may be experienced due to skin absorption. Runoff from fire control may be corrosive and/or toxic and cause pollution.
Health Hazard
White phosphorus is a highly toxic substance by all routes of exposure. Contact of the solid with the skin produces deep painful burns, and eye contact can cause severe damage. Ingestion of phosphorus leads (after a delay of a few hours) to symptoms including nausea, vomiting, belching, and severe abdominal pain. Apparent recovery may be followed by a recurrence of symptoms. Death may occur after ingestion of 50 to 100 mg due to circulatory, liver, and kidney effects. Phosphorus ignites and burns spontaneously when exposed to air, and the resulting vapors are highly irritating to the eyes and respiratory tract.
Red phosphorus is much less toxic than the white allotrope; however, samples of red phosphorus may contain the white form as an impurity. Early signs of chronic systemic poisoning by phosphorus are reported to include anemia, loss of appetite, gastrointestinal distress, chronic cough, a garlic-like odor to the breath, and pallor. A common response to severe chronic poisoning is damage of the jaw (''phossy jaw") and other bones. Phosphorus has not been reported to show carcinogenic effects in humans.
Health Hazard
White phosphorus is a highly poisonous substance. The toxic routes are ingestion, skin contact, and inhalation.
Inhumansasingleoraldoseof70–100 mg can cause death. The toxic symptoms are nausea, vomiting, severe abdominal pain, diarrhea, coma, and convulsions. The other harmful effects from ingestion are liver damage and jaundice. An amount as small as 5–10 mg of white phosphorus can exhibit some of the foregoingtoxic effectsinhumans from an oral intake. The lethal doses and symptoms for other species varied with the species. The toxic symptoms were somnolence, convulsion, and lung injury. The lethal doses ranges from 3 mg/kg for rats to 50 mg/kg for dogs.
Inhalation of its vapors can cause irritation of respiratory tract. The chronic poisoning from inhalation (or ingestion) severely affected the lungs, kidney, and liver in test animals. The toxic symptoms were bronchopneumonia, bone changes, necrosis of the jaw (“phossy” jaw), anemia, and weight loss. Since the vapor pressure of white phosphorus is low [0.026 torr at 20°C (68°F)], the acute health hazard from a short exposure to its vapors under normal conditions of its handling and uses should be low..
Fire Hazard
Extremely flammable; will ignite itself if exposed to air. Burns rapidly, releasing dense, white, irritating fumes. Substance may be transported in a molten form. May re-ignite after fire is extinguished. Corrosive substances in contact with metals may produce flammable hydrogen gas. Containers may explode when heated.
Fire Hazard
White phosphorus ignites spontaneously upon contact with air, producing an irritating, dense white smoke of phosphorus oxides. Use water to extinguish phosphorus fires.
Flammability and Explosibility
White phosphorus ignites spontaneously upon contact with air, producing an
irritating, dense white smoke of phosphorus oxides. Use water to extinguish
phosphorus fires.
Red phosphorus is a flammable solid but does not ignite spontaneously on exposure
to air. At high temperatures (-300 °C), red phosphorus is converted to the white form.
Agricultural Uses
Phosphorus (P) is an important nutrient for plants. It
is a non-metallic element having an atomic number
15. It belongs to Group 15 of the Periodic Table
. The use of phosphorus is as high as one tenth
of nitrogen.
Most plants contain phosphorus in concentrations
varying from 0.1 to 0.4%, which are considerably lower
than for potassium and nitrogen in plants. Phosphorus is
an essential part of nucleoproteins in cell nuclei which
control the cell division and the DNA molecules, the
latter transmitting heredity to living organisms.
Phosphorus also plays an important role in (a) stimulating
early root growth, (b) hastening plant maturity, (c)
transforming energy within the cells, and (d) developing
and ripening the fruit and the seed. Phosphorus is rightly
called the key to life, as it is directly involved in most life
processes.
Relations between phosphorus and N, Cu, Fe, Mn
and Zn are well known. Ratios of 3: 1 of N to P and 200: 1
of P to Zn are considered critical for addressing nutrient
deficiency in plants. The ratio of nitrogen to phosphorus
(N:P) serves as a Diagnosis and Recommendation
Integrated System (DRIS) norm for interpreting results
of plant analysis.
Soils have low total phosphate content and hence such
soils provide low supplies of available phosphate (400 to
2000 kg/ha) to plants because mineral phosphate forms
are not readily soluble. Plants absorb phosphorus as
H2PO4-; and HPO42- ions. On average, a soil solution
contains about 0.05 ppm phosphorus which varies from
soil to soil. This amount of phosphorus is adequate for
plants, as its concentration varies from 0.003 to 0.3 ppm
depending on the crop. For instance, maximum corn
yields are obtained at 0.01 ppm of the solutionphosphorus,
while the incorporation of solution
phosphorus in the case of wheat is only marginally more.
Soil phosphorus occurs in both organic and inorganic
forms. Plants differ in their ability to compete for soil
phosphorus at the growth stage when they need it most.
Young plants rapidly absorb phosphorus and accumulate
75 % of their requirement when the crop produces 25 % of
its dry weight. Winter wheat absorbs about 70% of
phosphorus between tillering and flowering. For corn,
the peak phosphorus demand is during the initial three
weeks of the growing season.
Safety Profile
Human poison by ingestion. Experimental poison by ingestion and subcutaneous routes. Experimental reproductive effects. Human systemic effects by ingestion: cardiomyopathy, cyanosis, nausea or vomiting, sweating. Toxic quantities have an acute effect on the liver and can cause severe eye damage. Inhalation can cause photophobia with myosis, dilation of the pupils, retinal hemorrhage, congestion of the blood vessels, and, rarely, an optic neuritis. Chronic exposure by inhalation or ingestion can cause anemia, gastrointestinal effects, and brittleness of the long bones, leading to spontaneous fractures. The most common symptom, however, of chronic phosphorus poisoning is necrosis of the jaw (phossyjaw). More reactive than red phosphorus. Dangerous fire hazard when exposed to heat, flame, or by chemical reaction with oxidtzers. Igmtes spontaneously in air. Very reactive. If combustion occurs in a confined space, it will remove the oxygen and cause asphyxiation. Dangerous explosion hazard by chemical reaction with: alkaline hydroxides, NH4NO3, SbF5, Ba(BrO3)2, Be, Bl3, Ca(BrO3)2, Mg(BrO3)2, K(BrO3), NaBrO3, Zn(BrO3)2, Br2, halogens, BrF3, BrN3, (chlorates of Ba, Ca, Mg, K, Na, Zn), (iodates of Ba, Ca, Mg, K, Na, Zn), Ce, Cs,CsHC2, CS3N, (charcoal + air), ClO2, (Ch + heptane), Cl0, ClF3, ClO3, chlorosulfonic acid, Cr03, Cr(OCl)2, Cu, NCl, IBr, ICl, IFj, Fe, La, PbO2, Li, LizC2, Li6CS, Mg(ClO4)z, Mn, HgO, HgNO3, Nd, Ni, nitrates, NBr, N02, NBr3, NCh, NOF, FN02, O2, performic acid, Pt, K, KOH, K3N, I(Mn04, K2O2, Rb, RbHC2, Se2Cl2, SeOCl2, SeOF2, SeF4, AgNO3, Ag20, Na, Na2C2, NaClO2, NaOH, Na2O2, S, so3, H2SO4, Th, VOCl2, Zr, peroxyformic acid, chloro sulfuric acid, halogen azides, hexalithum dtshcide. Can react vigorously with oxidtzing materials. To fight fire, use water. Used in fertilizers, tracer bullets, incendiaries manufacturing, rat poison, and gas analysis. When heated to decomposition it emits highly toxic fumes of POx. See also PHOSPHORUS (red).
Metabolism
Not Available
Storage
Work with white phosphorus should be conducted in a fume hood to prevent exposure by inhalation, and splash goggles and impermeable gloves should be worn at all times to prevent eye and skin contact. Phosphorus should be stored under water in secondary containers in areas separate from oxidizing agents and other incompatible substances.
Purification Methods
Purify white phosphorus by melting it under dilute H2SO4—dichromate (possible carcinogen) mixture and allow to stand for several days in the dark at room temperature. It remains liquid, and the initial milky appearance due to insoluble, oxidisable material gradually disappears. The phosphorus can then be distilled under vacuum in the dark [Holmes Trans Faraday Soc 58 1916 1962]. It sublimes in vacuo. Other methods of purification include extraction with dry CS2 followed by evaporation of the solvent, or washing with 6M HNO3, then H2O, and drying under vacuum. It ignites in air at ~50o, or by friction if dry. Store and cut it under H2O . POISONOUS.
Toxicity evaluation
Phosphorus is an oxidizing agent that, when exposed to air, may burn spontaneously. Thus, direct contact may result in both thermal and chemical burns. Second- and third-degree burns can be seen at the point of contact. When absorbed, phosphorus acts as a cellular poison by uncoupling oxidative phosphorylation. Red phosphorus is not considered to be potentially toxic as it is insoluble, nonvolatile, and unabsorbable.
Incompatibilities
White phosphorus reacts with a number of substances to form explosive mixtures.
For example, dangerous explosion hazards are produced upon reaction of
phosphorus with many oxidizing agents, including chlorates, bromates, and many
nitrates, with chlorine, bromine, peracids, organic peroxides, chromium trioxide, and
potassium permanganate, with alkaline metal hydroxides (phosphine gas is
liberated), and with sulfur, sulfuric acid, and many metals, including the alkali
metals, copper, and iron.
Red phosphorus is much less reactive than the white allotrope but may ignite or react
explosively with strong oxidizing agents.
Waste Disposal
Excess phosphorus and waste material containing this substance should be placed in an appropriate container, clearly labeled, and handled according to your institution's waste disposal guidelines.
Properties of Phosphorus
| Melting point: | 280 °C (white)(lit.) |
| Boiling point: | 280℃ |
| Density | 2.34 g/mL at 25 °C(lit.) |
| vapor density | 0.02 (vs air) |
| vapor pressure | 0.03 mm Hg ( 21 °C) |
| Flash point: | 30°C |
| storage temp. | 2-8°C |
| solubility | insoluble |
| form | powder (red) |
| color | Red-brown |
| Specific Gravity | 2.34 |
| PH | 3 at 37℃ and 500-10000mg/L |
| Odor | Acrid fumes when exposed to air |
| Resistivity | 10 μΩ-cm, 20°C |
| Water Solubility | insoluble |
| Merck | 13,7433 |
| Dielectric constant | 4.1(34℃) |
| Exposure limits | ACGIH: TWA 2 ppm; STEL 4 ppm OSHA: TWA 2 ppm(5 mg/m3) NIOSH: IDLH 25 ppm; TWA 2 ppm(5 mg/m3); STEL 4 ppm(10 mg/m3) |
| Stability: | Stable. Highly flammable. Incompatible with strong oxidizing agents, strong bases. Light and heat sensitive. |
| CAS DataBase Reference | 7723-14-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Phosphorus atom(7723-14-0) |
| EPA Substance Registry System | Phosphorus (7723-14-0) |
Safety information for Phosphorus
| Signal word | Warning |
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H228:Flammable solids H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P370+P378:In case of fire: Use … for extinction. |
Computed Descriptors for Phosphorus
Phosphorus manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
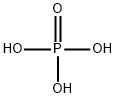
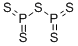


You may like
-
 Phosphorous Red CAS 7723-14-0View Details
Phosphorous Red CAS 7723-14-0View Details
7723-14-0 -
 Red phosphorus CASView Details
Red phosphorus CASView Details -
 Phosphorus powder, -100 mesh, red amorphous, 98.9% CAS 7723-14-0View Details
Phosphorus powder, -100 mesh, red amorphous, 98.9% CAS 7723-14-0View Details
7723-14-0 -
 Phosphorus Red pure 97% CAS 7723-14-0View Details
Phosphorus Red pure 97% CAS 7723-14-0View Details
7723-14-0 -
 Phosphorous red CAS 7723-14-0View Details
Phosphorous red CAS 7723-14-0View Details
7723-14-0 -
 PHOSPHORUS RED For Synthesis CAS 7723-14-0View Details
PHOSPHORUS RED For Synthesis CAS 7723-14-0View Details
7723-14-0 -
 Phosphorus, red CAS 7723-14-0View Details
Phosphorus, red CAS 7723-14-0View Details
7723-14-0 -
 Red Phosphorus, BagView Details
Red Phosphorus, BagView Details
7723-14-0
