Phosphoric acid
Synonym(s):o-Phosphoric acid, Orthophosphoric acid;Orthophosphoric acid;Orthophosphoric acid, o-Phosphoric acid;Phosphoric acid;Phosphoric acid solution
- CAS NO.:7664-38-2
- Empirical Formula: H3O4P
- Molecular Weight: 98
- MDL number: MFCD00011340
- EINECS: 231-633-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:40
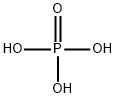
What is Phosphoric acid?
Absorption
Ortho phosphate is absorbed from, and to a limited extent secreted into, the gastrointestinal tract.
Description
The most common form of phosphoric acid (H3PO4) is the 85% aqueous form. Pure anhydrous phosphoric acid is a white solid with a melting point of 42 C. Phosphoric acid has three pKa values covering a wide range (pKa's: 2.15, 7.20, 12.32). This is the reason why phosphoric acid solutions make a great choice as a buffer.
Chemical properties
Phosphoric acid can appear as a colorless, odorless crystalline solid or a thick, syrupy liquid, with its physical state varying based on its strength and temperature. When concentrated, it is a colorless, odorless, syrupy liquid that, when diluted appropriately, has a pleasantly acidic taste. Pure phosphoric acid, also referred to as orthophosphoric acid, is a clear, colorless mineral acid of moderate strength. It is typically sold as an aqueous solution containing 75%-85% phosphoric acid, in which form it is a clear, viscous liquid.
Pharmacokinetics
Transport of phosphate from the gut lumen is an active, energy-dependent process that is modified by several factors. ... Vitamin D stimulates phosphate absorption, an effect reported to precede its action on calcium ion transport.
The Uses of Phosphoric acid
Phosphoric acid has a wide range of applications across various industries. It is used in the production of superphosphate fertilizers, livestock feeds, phosphate salts, polyphosphates, soaps, waxes, polishes, and detergents. Additionally, it serves as a soil stabilizer, in the manufacture of fire control agents, opal glasses, electric lights, and in processes such as cotton dyeing, tile cleaning, ceramic binding, dental cement, water treatment, electro-polishing, lithography, photoengraving, and process engraving. It is also utilized as a petrol additive, in coagulating rubber latex, and in metal rust proofing before painting. In chemical manufacturing, phosphoric acid acts as an acid catalyst for making ethylene and purifying hydrogen peroxide, as well as in the production of chemicals like ethylbenzene, propylene, and cumene. It is used as a bonding agent for refractory bricks, in extracting penicillin, and as an analytical agent. In the food industry, it functions as an antioxidant, a flavor additive for sharp taste in foods like jellies and soft drinks (e.g., Coca-Cola), and in the manufacture of yeasts and gelatin. Phosphoric acid is also used to manufacture phosphoric acid electrolyte fuel cell systems and has been applied in the treatment of lead poisoning.
Properties of Phosphoric acid
| Melting point: | ~40 °C(lit.) |
| Boiling point: | 158 °C(lit.) |
| Density | 1.685 g/mL at 25 °C(lit.) |
| vapor density | 3.4 (vs air) |
| vapor pressure | 2.2 mm Hg ( 20 °C) |
| refractive index | n |
| FEMA | 2900 | PHOSPHORIC ACID |
| storage temp. | no restrictions. |
| solubility | H2O: soluble |
| form | Solid or Viscous Liquid |
| appearance | Colorless, viscous liquid (85% aq) |
| pka | 2.1-7.2-12.3(at 25℃) |
| Specific Gravity | 1.7 |
| color | ≤10(APHA) |
| Odor | Odorless |
| PH Range | 1.5 |
| PH | 3.06(1 mM solution);2.26(10 mM solution);1.63(100 mM solution); |
| Water Solubility | MISCIBLE |
| λmax | λ: 260 nm Amax: ≤0.05 λ: 280 nm Amax: ≤0.04 |
| Merck | 14,7344 |
| BRN | 1921286 |
| Exposure limits | TLV-TWA 1 mg/m3 (ACGIH, MSHA, and
OSHA); TLV-STEL 3 mg/m3 (ACGIH). |
| Dielectric constant | 61.0 |
| CAS DataBase Reference | 7664-38-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Phosphoric acid(7664-38-2) |
| EPA Substance Registry System | Phosphoric acid (7664-38-2) |
Safety information for Phosphoric acid
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H290:Corrosive to Metals H302:Acute toxicity,oral H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P234:Keep only in original container. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Phosphoric acid
| InChIKey | NBIIXXVUZAFLBC-UHFFFAOYSA-N |
Phosphoric acid manufacturer
JSK Chemicals
Voda Chemicals Private Limited
NRS Chemicals LLP
Royal Agri Chemicals And Fertilizers Private Limited
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Phosphoric Acid 98%View Details
Phosphoric Acid 98%View Details -
 Phosphoric Acid 99%View Details
Phosphoric Acid 99%View Details -
 Phosphoric Acid 99%View Details
Phosphoric Acid 99%View Details -
 Phosphoric Acid (H3PO4) 99%View Details
Phosphoric Acid (H3PO4) 99%View Details -
 Phosphoric Acid CASView Details
Phosphoric Acid CASView Details -
 Phosphoric acid CASView Details
Phosphoric acid CASView Details -
 Extran® AP 21 CASView Details
Extran® AP 21 CASView Details -
 Phosphorus ICP standard CASView Details
Phosphorus ICP standard CASView Details
