Phosphorus pentasulfide
Synonym(s):Diphosphorus pentasulfide, Phosphorus(V) sulfide;Phosphorus pentasulfide;Phosphorus(V) sulfide
- CAS NO.:1314-80-3
- Empirical Formula: PS5
- Molecular Weight: 191.3
- MDL number: MFCD00011441
- EINECS: 215-242-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
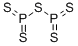
What is Phosphorus pentasulfide?
Description
Phosphorus pentasulfide, is a nonmetallic inorganic compound . It is a yellow to greenish-yellow crystalline mass with an odor similar to hydrogen sulfide. It is a dangerous fire risk and ignites by friction or in contact with water. Boiling point is 995°F (535°C) and ignition temperature is 287°F (141°C). It decomposes upon contact with water or moist air, liberating toxic and flammable hydrogen-sulfide gas. Specific gravity is 2.09, so it is heavier than water. It is toxic by inhalation, with a TLV of 1 mg/m3 of air. The four-digit UN identification number is 1340. The NFPA 704 designation is health 2, flammability 1, and reactivity 2. Primary uses are in insecticides, safety matches, ignition compounds, and sulfonation.
Chemical properties
Phosphorus pentasulfide is a greenish-gray to yellow, crystalline solid with an odor of rotten eggs. The Odor Threshold is 0.005 ppm.

The Uses of Phosphorus pentasulfide
Phosphorus pentasulfide is used in the manufacture of lubricant additives, pesticides, safety matches, and flotation agents.
The Uses of Phosphorus pentasulfide
In manufacture of lube oil additives and pesticides. manufacture of safety matches, ignition Compounds, and for introducing sulfur into organic Compounds.
The Uses of Phosphorus pentasulfide
Phosphorus pentasulfide (phosphoric sulfide, P2S5) is an insecticide. It is also an additive to oils and a component of safety matches.
General Description
A greenish yellow solid with an odor of rotten eggs that may paralyze the sense of smell at hazardous concentrations in air. Density 2.04 g / cm3. Phosphorus pentasulfide is used for making lube oil additives, insecticides, flotation agents, safety matches, blown asphalt, and other products and chemicals.
Air & Water Reactions
Highly flammable. May heat and spontaneously ignite in presence of moisture [Haz. Chem. Data. 1969]. Reaction with water forms toxic hydrogen sulfide gas and phosphoric acid. Dangerous when wet.
Reactivity Profile
PHOSPHORUS PENTASULFIDE reacts vigorously with strong oxidants. Exothermically violent decomposition reaction with water, steam or acids to produce irritating fumes of phosphorus pentaoxide and highly toxic hydrogen sulfide gas. May ignite in contact with limited amounts of water [Haz. Chem. Data, 1975, p. 239]. Can be ignited by sparks or friction and its dust presents an explosion hazard in air at sufficient concentrations. Hydrogen sulfide gas evolved from reactions with water may also form explosive mixtures with air.
Health Hazard
Phosphorus pentasulfide is an irritant to the skin and eyes. Inhalation of its vapors can cause irritation of the respiratory passage. Oral toxicity of this compound in rats was found to be moderate.
LD50 value, oral (rats): 389 mg/kg (NIOSH 1986)
Phosphorus pentasulfide can readily produce highly toxic hydrogen sulfide in the presence of moisture. Other toxic products from its combustion are sulfur dioxide and phosphorus pentoxide (corrosive)..
Fire Hazard
Special Hazards of Combustion Products: Products of combustion include sulfur dioxide and phosphorus pentoxide, which are irritating, toxic and corrosive.
Flammability and Explosibility
Highly flammable
Safety Profile
A poison by ingestion. A severe eye and skin irritant. Readily liberates toxic hydrogen sulfide and phosphorus pentoxide and evolves heat on contact with moisture. Dangerous fire hazard in the form of dust when exposed to heat or flame. Spontaneous heating in the presence of moisture. Moderate explosion hazard in solid form by spontaneous chemical reaction. Reacts with water, steam, or acids to produce toxic and flammable vapors; can react vigorously with oxidizing materials. Incompatible with air, alcohols, water. To fight fire, use CO2snow, dry chemical, or sand. Used as an intermedate in manufacturing lubricant addltives, insecticides, and fertilizer agents. When heated to decomposition it emits highly toxic fumes of SO, and PO,. See also HYDROGEN SULFIDE.
Potential Exposure
Phosphorus pentasulfide is used as an intermediate in the manufacture of lubricant additives; insecticides, flotation agents; lubricating oil; ignition compounds; and matches. It is also used to introduce sulfur into rubber, and organic chemicals, such as pharmaceuticals.
Shipping
UN1340 Phosphorus pentasulfate, free from yellow or white phosphorus, Hazard Class: 4.3; Labels: 4.3-Dangerous when wet material, 4.1-flammable solid.
Purification Methods
Purify P2S5 by extraction and crystallisation with CS2, using a Soxhlet extractor, and is heated in a CO2 atmosphere at 150o to remove solvent. It liberates H2S in moist air. HARMFUL VAPOURS. [Klements in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 568 1963.]
Incompatibilities
Flammable solid; dust may form explosive mixture with air. Contact with water forms phosphorus pentoxide and an explosive mixture of hydrogen sulfide with air. Phosphorus pentasulfide is incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, ammonia, strong acids, strong bases, alcohols. Reaction with water or moisture in the air releases heat, hydrogen sulfide (H2S), sulfur dioxide, and phosphoric acid. Pyrophoric hazard, may self-ignite in moist air.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Decompose with water, forming phosphoric acid, sulfuric acid and hydrogen sulfide. Provisions must be made for scrubbing hydrogen sulfide emissions. The acids may then be neutralized and diluted slowly to solution of soda ash and slaked lime with stirring, then flush to sewer with large volumes of water.
Properties of Phosphorus pentasulfide
| Melting point: | 286 °C |
| Boiling point: | 514°C |
| Density | 2.09 g/mL at 25 °C(lit.) |
| storage temp. | Flammables area |
| solubility | reacts with H2O; soluble in CS2 |
| appearance | Greenish-gray to yellow solid |
| form | Powder |
| color | Yellow to green |
| PH | 1 (10g/l, H2O, 20℃) |
| Odor | rotten egg odor |
| Water Solubility | reacts |
| Stability: | Hygroscopic, Moisture Sensitive |
| CAS DataBase Reference | 1314-80-3(CAS DataBase Reference) |
| EPA Substance Registry System | Phosphorus pentasulfide (1314-80-3) |
Safety information for Phosphorus pentasulfide
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H228:Flammable solids H260:Substances And Mixtures Which, In Contact With Water,Emit Flammable Gases H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P223:Keep away from any possible contact with water, because of violent reaction and possible flash fire. P273:Avoid release to the environment. P231+P232:Handle under inert gas. Protect from moisture. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
Computed Descriptors for Phosphorus pentasulfide
| InChIKey | HWVAJUNEPOKXLF-UHFFFAOYSA-N |
Phosphorus pentasulfide manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran




You may like
-
 Phosphorus pentasulfide 98%View Details
Phosphorus pentasulfide 98%View Details -
 1314-80-3 99%View Details
1314-80-3 99%View Details
1314-80-3 -
 Phosphorous pentasulphide CAS 1314-80-3View Details
Phosphorous pentasulphide CAS 1314-80-3View Details
1314-80-3 -
 Phosphorous pentasulphide CAS 1314-80-3View Details
Phosphorous pentasulphide CAS 1314-80-3View Details
1314-80-3 -
 PHOSPHORUS PENTASULPHIDE For Synthesis CAS 1314-80-3View Details
PHOSPHORUS PENTASULPHIDE For Synthesis CAS 1314-80-3View Details
1314-80-3 -
 YELLOW CRYSTAL Phosphorus Pentasulphide CAS Number 1314-80-3View Details
YELLOW CRYSTAL Phosphorus Pentasulphide CAS Number 1314-80-3View Details
1314-80-3 -
 Phosphorus Pentasulphide .View Details
Phosphorus Pentasulphide .View Details
1314-80-3 -
 Phosphorus PentasulphideView Details
Phosphorus PentasulphideView Details
1314-80-3
