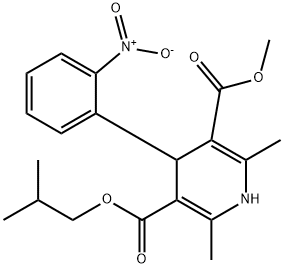
Nisoldipine
Synonym(s):(±)-Isobutyl methyl 1,4-dihydro-2,6-dimethyl-4-(o-nitrophenyl)-3,5-pyridinedicarboxylate;1,4-Dihydro-2,6-dimethyl-4-(2-nitrophenyl)-3,5-pyridinedicarboxylic acid methyl 2-methylpropyl ester;Sular
- CAS NO.:63675-72-9
- Empirical Formula: C20H24N2O6
- Molecular Weight: 388.41
- MDL number: MFCD00478055
- EINECS: 264-407-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
What is Nisoldipine?
Absorption
Relatively well absorbed into the systemic circulation with 87% of the radiolabeled drug recovered in urine and feces. The absolute bioavailability of nisoldipine is about 5%.
Description
Nisoldipine D4 is another long-acting dihydropyridine calcium channel blocker effective in the treatment of hypertension and angina pectoris. It is reported to have little or no cardiodepressive activity and to be more effective than nifedipine in reducing blood pressure in hypertensives. Nisoldipine has also been shown to be effective in the management of congestive heart failure. Reduction in dosage is necessary in patients with hepatic, but not renal impairment.
Chemical properties
Nisoldipine D4 is Light Tan Crystals
Originator
Bayer AG (Germany)
The Uses of Nisoldipine
Labeled Nisoldipine, intended for use as an internal standard for the quantification of Nisoldipine by GC- or LC-mass spectrometry.
The Uses of Nisoldipine
Dihydropyridine calcium channel blocker. Antihypertensive and antianginal
The Uses of Nisoldipine
urease inhibitor
Indications
For the treatment of hypertension. It may be used alone or in combination with other antihypertensive agents.
Background
Nisoldipine is a 1,4-dihydropyridine calcium channel blocker. It acts primarily on vascular smooth muscle cells by stabilizing voltage-gated L-type calcium channels in their inactive conformation. By inhibiting the influx of calcium in smooth muscle cells, nisoldipine prevents calcium-dependent smooth muscle contraction and subsequent vasoconstriction. Nisoldipine may be used in alone or in combination with other agents in the management of hypertension.
What are the applications of Application
Nisoldipine is a calcium channel protein inhibitor
Definition
ChEBI: A dihydropyridine that is 1,4-dihydropyridine which is substituted by methyl groups at positions 2 and 6, a methoxycarbonyl group at position 3, an o-nitrophenyl group at position 4, and an isobutoxycarbonyl group at position 5. The racemate, calcium channel blocker, is used in the treatment of hypertension and angina pectoris.
Manufacturing Process
Boiling a solution of 12.7 g of 2'-nitrobenzylideneacetoacetic acid methyl ester and 7.1 g of β-amino-crotonic acid isopropyl ester in 50 ml of methanol for 10 hours yielded 2,6-dimethyl-4-(2'-nitrophenyl)-1,4-dihydropyridine-3,5- dicarboxylic acid 3-methyl ester-5-isopropyl ester of melting point 174°C (from ethanol). Yield 48% of theory.
brand name
Sular (Sciele);Baymycard.
Therapeutic Function
Coronary vasodilator
General Description
In vitro studies show that the effects ofnisoldipine, 1,4-dihydro-2, 6-dimethyl-4-(2-nitrophenyl)-3,5-pyridinecarboxylic acid methyl 2-methylpropyl ester(Sular), on contractile processes are selective, with greaterpotency on vascular smooth muscle than on cardiac muscle.
Nisoldipine is highly metabolized, with five majormetabolites identified. As with most of the dihydropyridines,the cytochrome P450 (CYP) 3A4 isozyme is mainlyresponsible for the metabolism of nisoldipine. The majorbiotransformation pathway appears to involve the hydroxylationof the isobutyl ester side chain. This particular metabolitehas approximately 10% of the activity of the parentcompound.
Biochem/physiol Actions
L-type (CaV1.2) calcium channel blocker; dihydropyridine-type calcium channel blocker with selectivity for smooth muscle (CaV1.2b) over cardiac muscle (CaV1.2a). Arterial vasodilator and antihypertensive agent.
Pharmacokinetics
Nisoldipine, a dihydropyridine calcium-channel blocker, is used alone or with an angiotensin-converting enzyme inhibitor, to treat hypertension, chronic stable angina pectoris, and Prinzmetal's variant angina. Nisoldipine is similar to other peripheral vasodilators. Nisoldipine inhibits the influx of extra cellular calcium across the myocardial and vascular smooth muscle cell membranes possibly by deforming the channel, inhibiting ion-control gating mechanisms, and/or interfering with the release of calcium from the sarcoplasmic reticulum. The decrease in intracellular calcium inhibits the contractile processes of the myocardial smooth muscle cells, causing dilation of the coronary and systemic arteries, increased oxygen delivery to the myocardial tissue, decreased total peripheral resistance, decreased systemic blood pressure, and decreased afterload.
Metabolism
Pre-systemic metabolism in the gut wall, and this metabolism decreases from the proximal to the distal parts of the intestine. Nisoldipine is highly metabolized; 5 major urinary metabolites have been identified. The major biotransformation pathway appears to be the hydroxylation of the isobutyl ester. A hydroxylated derivative of the side chain, present in plasma at concentrations approximately equal to the parent compound, appears to be the only active metabolite and has about 10% of the activity of the parent compound. Cytochrome P450 enzymes are believed to play a major role in the metabolism of nisoldipine. The particular isoenzyme system responsible for its metabolism has not been identified, but other dihydropyridines are metabolized by cytochrome P450 IIIA4.
Properties of Nisoldipine
| Melting point: | 147-148°C |
| Boiling point: | 503.3±50.0 °C(Predicted) |
| Density | 1.205±0.06 g/cm3(Predicted) |
| storage temp. | room temp |
| solubility | DMSO: >10mg/mL |
| form | powder |
| pka | 2.67±0.70(Predicted) |
| color | yellow |
| Merck | 14,6565 |
| CAS DataBase Reference | 63675-72-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Nisoldipine(63675-72-9) |
Safety information for Nisoldipine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for Nisoldipine
| InChIKey | VKQFCGNPDRICFG-UHFFFAOYSA-N |
Nisoldipine manufacturer
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran

You may like
-
 63675-72-9 Nisoldipine 98%View Details
63675-72-9 Nisoldipine 98%View Details
63675-72-9 -
 63675-72-9 98%View Details
63675-72-9 98%View Details
63675-72-9 -
 Nisoldipine >98% (HPLC) CAS 63675-72-9View Details
Nisoldipine >98% (HPLC) CAS 63675-72-9View Details
63675-72-9 -
 Nisoldipine CAS 63675-72-9View Details
Nisoldipine CAS 63675-72-9View Details
63675-72-9 -
 Nisoldipine 99%View Details
Nisoldipine 99%View Details
63675-72-9 -
 Nisoldipine 63675-72-9 98%View Details
Nisoldipine 63675-72-9 98%View Details
63675-72-9 -
 Nisoldipine CAS 63675-72-9View Details
Nisoldipine CAS 63675-72-9View Details
63675-72-9 -
 Nisoldipine CASView Details
Nisoldipine CASView Details
