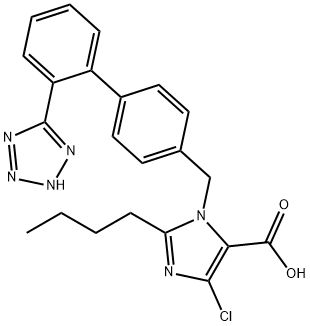
Losartan carboxylic acid
Synonym(s):2-Butyl-4-chloro-1-[[2′-(2H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-1H-imidazole-5-carboxylic acid;E-3174;EXP 3174;EXP3174;EXP-3174
- CAS NO.:124750-92-1
- Empirical Formula: C22H21ClN6O2
- Molecular Weight: 436.89
- MDL number: MFCD00871928
- EINECS: 1592732-453-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
What is Losartan carboxylic acid?
Description
Losartan carboxylic acid is a physiologically active metabolite of losartan , produced by cytochrome P450 isoforms in the liver. Like the parent compound, losartan carboxylic acid is a potent AT1 antagonist (Kis = 0.57 and 0.67 nM for rat and human forms, respectively), producing a depressor response and vasodilatation. When administered intravenously, losartan carboxylic acid is more potent and has a longer duration of action than losartan. However, the metabolite has very low oral bioavailability. Losartan, but not its metabolite, inhibits platelet aggregation in vitro.
Chemical properties
Light-Yellow Solid
The Uses of Losartan carboxylic acid
Losartan carboxylic acid is used as a metabolite of Losartan.
What are the applications of Application
Losartan carboxylic acid is a metabolite of Losartan
Definition
ChEBI: A biphenylyltetrazole that is losartan with the hydroxymethyl group at position 5 on the imidazole ring replaced with a carboxylic acid.
in vitro
e-3174 potently blocked the specific binding of [125i]-aii to vsmc isolated from rat aorta. e-3174 was able to dampen the platelet-derived growth factor-induced increase in cell dna synthesis and protein, which led to the blockade of the aii-induced increase in cell protein [1].
in vivo
rats were administrated e-3174 intravenously at a dose of l.0 mg/kg. after 6 hours, e-3174 markedly attenuated the cardiovascular effects of aii in rats. e-3174 induced a progressive fall in mean arterial pressure and a marked increase in renal flow only [2].
Storage
Store at -20°C
References
[1]. li, x. & widdop, r. angiotensin type i receptor antagonists cy-11974 and exp 3174 cause selective renal vasodilatation in conscious spontaneously hypertensive rats. clinical science, 1996; 91(2): 147-154.
[2]. sachinidis, a., ko, y., weisser, p., zu bricbkwedde, m., dsing, r., & christian, r. et al. exp3174, a metabolite of losartan (mk954, dup753) is more potent than losartan in blocking the angiotensin ll-induced responses in vascular smooth muscle cells. journal of hypertension. 1993; 11(2): 155-162.
Properties of Losartan carboxylic acid
| Melting point: | 130-132°C |
| Boiling point: | 707.8±70.0 °C(Predicted) |
| Density | 1.41±0.1 g/cm3(Predicted) |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | Dimethylformamide, Dimethyl Sulfoxide, Methanol |
| form | Solid |
| pka | 0.79±0.50(Predicted) |
| color | Light-Yellow |
Safety information for Losartan carboxylic acid
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H362:Reproductive toxicity, effects on or via lactation |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P263:Avoid contact during pregnancy/while nursing. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Losartan carboxylic acid
| InChIKey | ZEUXAIYYDDCIRX-UHFFFAOYSA-N |
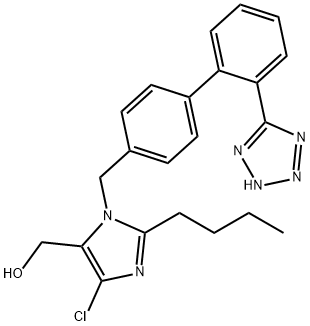
| SMILES | C1(CCCC)N(CC2=CC=C(C3=CC=CC=C3C3=NNN=N3)C=C2)C(C(O)=O)=C(Cl)N=1 |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 124750-92-1 Losartan Carboxylic Acid 96.33View Details
124750-92-1 Losartan Carboxylic Acid 96.33View Details
124750-92-1 -
 Losartan carboxylic acid CAS 124750-92-1View Details
Losartan carboxylic acid CAS 124750-92-1View Details
124750-92-1 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
