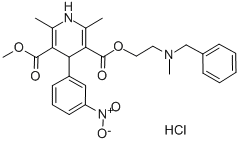
Nicardipine
- CAS NO.:55985-32-5
- Empirical Formula: C26H29N3O6
- Molecular Weight: 479.53
- MDL number: MFCD00216027
- EINECS: 259-932-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-20 11:41:24
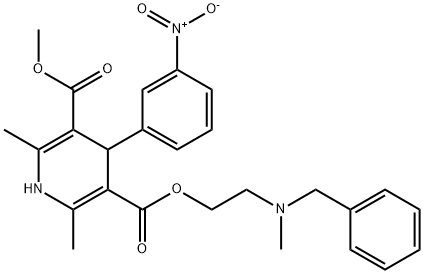
What is Nicardipine?
Absorption
While nicardipine is completely absorbed, it is subject to saturable first pass metabolism and the systemic bioavailability is about 35% following a 30 mg oral dose at steady state.
Toxicity
Oral LD50 Rat = 184 mg/kg, Oral LD50 Mouse = 322 mg/kg
Originator
Nicodel,Mitsui,Japan,1981
The Uses of Nicardipine
Nicardipine is a dihydropyridine calcium channel blocker. Antianginal; antihypertensive. Neuroprotective & Neuroresearch products.
The Uses of Nicardipine
Vasodilator.
The Uses of Nicardipine
It is used for arterial hypertension, chronic, stable angina pectoris, preventing angina pectoris, and for ischemic-type abnormalities of brain blood flow.
Background
A potent calcium channel blockader with marked vasodilator action. It has antihypertensive properties and is effective in the treatment of angina and coronary spasms without showing cardiodepressant effects. It has also been used in the treatment of asthma and enhances the action of specific antineoplastic agents. [PubChem]
Indications
Used for the management of patients with chronic stable angina and for the treatment of hypertension.
Definition
ChEBI: Nicardipine is a racemate comprising equimolar amounts of (R)- and (S)-nicardipine. It is a calcium channel blocker which is used to treat hypertension. It has a role as an antihypertensive agent, a calcium channel blocker, a vasodilator agent and an autophagy inhibitor. It contains a (S)-nicardipine and a (R)-nicardipine.
Manufacturing Process
A mixture of 4.98 g of acetoacetic acid N-benzyl-N-methylaminoethyl ester, 2.3 g of β-aminocrotonic acid methyl ester, and 3 g of m-nitrobenzaldehyde was stirred for 6 hours at 100°C in an oil bath. The reaction mixture was subjected to a silica gel column chromatography (diameter 4 cm and height 25 cm) and then eluted with a 20:1 mixture of chloroform and acetone. The effluent containing the subject product was concentrated and checked by thin layer chromatography. The powdery product thus obtained was dissolved in acetone and after adjusting the solution with an ethanol solution saturated with hydrogen chloride to pH 1-2, the solution was concentrated to provide 2 g of 2,6-dimethyl-4-(3'-nitrophenyl)1,4-dihydropyridine-3,5-dicarboxylic acid 3-methylester-5-β-(N-benzyl-N-methylamino)ethyl ester hydrochloride. The product thus obtained was then crystallized from an acetone mixture, melting point 136°C to 140°C (decomposed).
brand name
Cardene(PDL Biopharma); Cardene (Roche).
Therapeutic Function
Vasodilator
Mechanism of action
Nicardipin relaxes smooth musculature of vessels, lowers resistance of coronary and peripheral vessels, increases blood flow in vessels of the brain, causes a moderate and stable hypotensive effect, and reduces the myocardial need for oxygen.
Pharmacokinetics
Nicardipine, a dihydropyridine calcium-channel blocker, is used alone or with an angiotensin-converting enzyme inhibitor, to treat hypertension, chronic stable angina pectoris, and Prinzmetal's variant angina. Nicardipine is similar to other peripheral vasodilators. Nicardipine inhibits the influx of extra cellular calcium across the myocardial and vascular smooth muscle cell membranes possibly by deforming the channel, inhibiting ion-control gating mechanisms, and/or interfering with the release of calcium from the sarcoplasmic reticulum. The decrease in intracellular calcium inhibits the contractile processes of the myocardial smooth muscle cells, causing dilation of the coronary and systemic arteries, increased oxygen delivery to the myocardial tissue, decreased total peripheral resistance, decreased systemic blood pressure, and decreased afterload.
Synthesis
Nicardipine, 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-methyl-2-[(methylphenylmethyl)- amino]ethyl ester 3,5-pirididincarboxylic acid (19.3.7), is synthesized in a manner analogous to the synthesis of nifedipine, the only difference being that in the Hantsch synthesis, two different |?-dicarbonyl compounds are used simultaneously with o-nitrobenzaldehyde. During this, one of these in the enamine form of acetoacetic ester is simultaneously used as an amine component. A heterocyclization reaction is accomplished by reacting, the methyl ester of |?-aminocrotonic acid with the 2-methyl-2-benzylaminoethyl ester of acetoacetic acid.
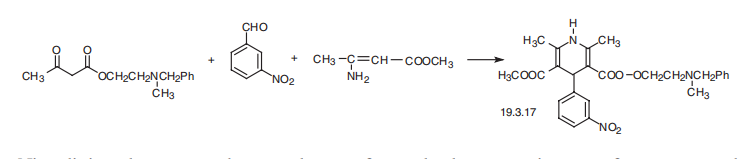
Metabolism
Nicardipine HCl is metabolized extensively by the liver.
Properties of Nicardipine
| Melting point: | 136-138 °C |
| Boiling point: | 603.4±55.0 °C(Predicted) |
| Density | 1.230±0.06 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | Dichloromethane; Methanol |
| form | Solid |
| pka | 7.30±0.50(Predicted) |
| color | Yellow |
| CAS DataBase Reference | 55985-32-5(CAS DataBase Reference) |
Safety information for Nicardipine
Computed Descriptors for Nicardipine
Nicardipine manufacturer
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran
You may like
-
 55985-32-5 NICARDIPINEView Details
55985-32-5 NICARDIPINEView Details
55985-32-5 -
 55985-32-5 98%View Details
55985-32-5 98%View Details
55985-32-5 -
 Nicardipine 98%View Details
Nicardipine 98%View Details -
 Nicardipine 55985-32-5 98%View Details
Nicardipine 55985-32-5 98%View Details
55985-32-5 -
 Nicardipine 95% CAS 55985-32-5View Details
Nicardipine 95% CAS 55985-32-5View Details
55985-32-5 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
