Doxazosin
- CAS NO.:74191-85-8
- Empirical Formula: C23H25N5O5
- Molecular Weight: 451.48
- MDL number: MFCD00800482
- EINECS: 616-059-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 16:15:04
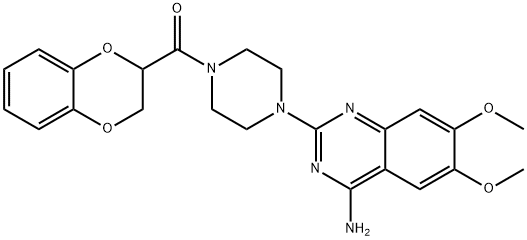
What is Doxazosin?
Absorption
Doxazosin is rapidly absorbed in the gastrointestinal tract and peak concentrations are achieved within 2-3 hours after administration. The bioavailability is about 60%-70%. The intake of food with doxazosin is not expected to cause clinically significant effects.
Toxicity
LD50 information
The oral LD50 of doxazosin in mice is >1000 mg/kg.
Overdose information
Symptoms of overdose include hypotension, changes in heart rate, and drowsiness. Administer supportive treatment in case of an overdose with doxazosin. Remove unabsorbed doxazosin from the gastrointestinal tract, correct hypotension, and closely monitor vital signs.
Description
Doxazosin mesylate is a selective alpha, blocker indicated for the treatment of hypertension, reportedly of special benefit as a first-line agent in the most hypertonic patient population. Another advantage of doxazosin is its favorable effect on blood lipids; it significantly increases the HDL/total cholesterol ratio and decreases the total cholesterol as well as triglycerides levels.
Originator
Pfizer (United Kingdom)
The Uses of Doxazosin
ACIPHEX therapeutic Erosive or Ulcerative Gastroesophageal Reflux Disease
Indications
Doxazosin is indicated to treat the symptoms of benign prostatic hypertrophy, which may include urinary frequency, urgency, and nocturia, among other symptoms. In addition, doxazosin is indicated alone or in combination with various antihypertensive agents for the management of hypertension. Off-label uses of doxazosin include the treatment of pediatric hypertension and the treatment of ureteric calculi.
Background
Doxazosin is an alpha-1 antagonist used for the treatment of benign prostatic hypertrophy (BPH) symptoms and hypertension. Other members of this drug class include Prazosin, Terazosin, Tamsulosin, and Alfuzosin. Because of its long-lasting effects, doxazosin can be administered once a day. It is marketed by Pfizer and was initially approved by the FDA in 1990.
What are the applications of Application
Doxazosin is a prazosin-related compound that is a selective α-1-adrenergic blocker
Definition
ChEBI: A member of the class of quinazolines that is quinazoline substituted by an amino group at position 4, methoxy groups at positions 6 and 7 and a piperazin-1-yl group at position 2 which in turn is substituted by a 2,3-dihydro-1,4-benzodioxin-2-ylcarbonyl roup at position 4. An antihypertensive agent, it is used in the treatment of high blood pressure.
brand name
Cardura (Pfizer);Carduran.
General Description
Doxazosin, 1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-(1,4-benzodioxan-2-ylcarbonyl)piperazine(Cardura), is a quinazoline compound that selectively inhibitsthe α1-subtype of α-adrenergic receptors. This agentis very useful in the management of hypertension associatedwith pheochromocytoma.
Pharmacokinetics
Doxazosin decreases standing and supine blood pressure and relieves the symptoms of benign prostatic hypertrophy through the inhibition of alpha-1 receptors.
Doxazosin may cause hypotension due to its pharmacological actions. This frequently occurs in the upright position, leading to a feeling of dizziness or lightheadedness. The first dose of doxazosin may lead to such effects, however, subsequent doses may also cause them. The risk of these effects is particularly high when dose adjustments occur or there are long intervals between doxazosin doses. Treatment should be started with the 1 mg dose of doxazosin, followed by slow titration to the appropriate dose. Patients must be advised of this risk and to avoid situations in which syncope and dizziness could be hazardous following the ingestion of doxazosin. Interestingly doxazosin exerts beneficial effects on plasma lipids. It reduces LDL (low-density lipoprotein) cholesterol and triglyceride levels and increases HDL (high-density lipoprotein) cholesterol levels.
A note on priapism risk
In rare cases, doxazosin and other alpha-1 blockers may cause priapism, a painful occurrence of persistent and unrelievable penile erection that can lead to impotence if medical attention is not sought as soon as possible. Patients must be advised of the priapism risk associated with doxazosin and to seek medical attention immediately if it is suspected.
Clinical Use
Alpha-adrenoceptor blocker:
Hypertension
Benign prostatic hyperplasia (BPH)
Drug interactions
Potentially hazardous interactions with other drugs
Antidepressants: enhanced hypotensive effect with
MAOIs.
Avanafil, vardenafil, sildenafil and tadalafil: enhanced
hypotensive effect, avoid with tadalafil, start the
others at the lowest possible dose.
Beta-blockers: enhanced hypotensive effect;
increased risk of first dose hypotensive effect.
Calcium-channel blockers: enhanced hypotensive
effect, increased risk of first dose hypotensive effect.
Diuretics: enhanced hypotensive effect, increased risk
of first dose hypotensive effect.
Moxisylyte: possibly severe postural hypotension
when used in combination.
Metabolism
Hepatic metabolism of doxazosin produces inactive O-demethylated and C-hydroxylated metabolites. Metabolism occurs via O-demethylation of the quinazoline nucleus of doxazosin or via hydroxylation of its benzodioxan portion. The enzymes involved in the metabolism of doxazosin include CYP2C19, CYP2D6, CYP2C19, and CYP3A4, which is the primary metabolizing enzyme. Doxazosin itself is considered to be mainly responsible for its pharmacological action, however, some active metabolites have been identified whose pharmacokinetics have not been adequately characterized.
Metabolism
Doxazosin is extensively metabolised in the liver, and excreted in faeces as inactive metabolites (6-hydroxydoxazosin) and a small amount of unchanged drug
Properties of Doxazosin
| Melting point: | 289-290°C |
| Boiling point: | 718.0±70.0 °C(Predicted) |
| Density | 1.371±0.06 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO |
| form | Powder |
| pka | 6.52±0.50(Predicted) |
| CAS DataBase Reference | 74191-85-8(CAS DataBase Reference) |
Safety information for Doxazosin
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H314:Skin corrosion/irritation H373:Specific target organ toxicity, repeated exposure H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P310:Immediately call a POISON CENTER or doctor/physician. P363:Wash contaminated clothing before reuse. P391:Collect spillage. Hazardous to the aquatic environment P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Doxazosin
Doxazosin manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
 74191-85-8 Doxazosin Mesylate 99%View Details
74191-85-8 Doxazosin Mesylate 99%View Details
74191-85-8 -
 Doxazosin 99%View Details
Doxazosin 99%View Details -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
