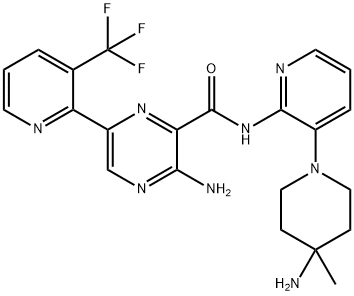
Infigratinib
- CAS NO.:872511-34-7
- Empirical Formula: C26H31Cl2N7O3
- Molecular Weight: 560.48
- MDL number: MFCD22123241
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
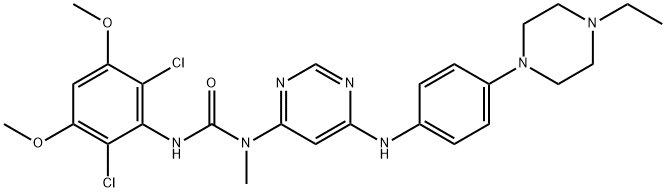
What is Infigratinib?
Absorption
Mean (%CV) Cmax is 282.5 ng/mL (54%) and AUC0-24h is 3780 ngxh/mL (59%) for infigratinib. Infigratinib Cmax and AUC increase more than proportionally across the dose range of 5 to 150 mg and steady state is achieved within 15 days. At steady state, median time to achieve peak infigratinib plasma concentration (Tmax) is six hours, with a range between two and seven hours.
Mean (%CV) Cmax is 42.1 ng/mL (65%) for BHS697 and 15.7 ng/mL (92%) for CQM157. Mean (%CV) AUC0-24h is 717 ngxh/mL (55%) for BHS697 and 428 ngxh/mL (72%) for CQM157. In healthy subjects, a high-fat and high-calorie meal increased AUCinf of infigratinib by 80%-120% and Cmax by 60%-80%. The median Tmax also shifted from four hours to six hours. A low-fat low-calorie meal increased the mean AUCinf of infigratinib by 70% and Cmax by 90%/
Toxicity
There is limited information on lethal doses and overdose of infigratinib. In clinical trials, infigratinib was associated with ocular toxicity (retinal pigment epithelial detachment), hyperphosphatemia leading to soft tissue mineralization, and embryo-fetal toxicity.
Description
NVP-BGJ398 (872511-34-7) is a potent and selective pan-FGFR inhibitor (IC50?= 0.9nM, 1.4 nM, 1.0 nM, and 60 nM for FGFR1,2,3,4 respectively).1? It has also been used in a mouse model of Achondroplasia (most common form of dwarfism) to correct pathological hallmarks of this condition.
The Uses of Infigratinib
BGJ 398 is a potent and selective inhibitor of the fibroblast growth factor receptor family of receptor tyrosine kinase.
Indications
Infigratinib is indicated for the treatment of previously treated, unresectable locally advanced or metastatic cholangiocarcinoma in adults with a fibroblast growth factor receptor 2 (FGFR2) fusion or another rearrangement as detected by an FDA-approved test.
Background
Infigratinib is a pan-fibroblast growth factor receptor (FGFR) kinase inhibitor. By inhibiting the FGFR pathway, which is often aberrated in cancers such as cholangiocarcinoma, infigratinib suppresses tumour growth. Cholangiocarcinoma is the most common primary malignancy affecting the biliary tract and the second most common primary hepatic malignancy. Infitratinib is a pan-FGFR inhibitor, as it is an ATP-competitive inhibitor of all four FGFR receptor subtypes.
On May 28, 2021, the FDA granted accelerated approval to infigratinib - under the market name Truseltiq - for the treatment of previously treated, unresectable locally advanced or metastatic cholangiocarcinoma in adults with a fibroblast growth factor receptor 2 (FGFR2) fusion or another rearrangement as detected by an FDA-approved test. This approval follows pemigatinib, another FGFR inhibitor approved by the FDA for the same therapeutic indication.
What are the applications of Application
BGJ398 is a potent and selective inhibitor of Flg (FGFR-1), Bek (FGFR-2), FGFR-3 and FGFR-4
Definition
ChEBI: BGJ-398 is a member of the class of phenylureas that is urea in which a hydrogen attached to one of the nitrogens is replaced by a 2,6-dichloro-3,5-dimethoxyphenyl group, while the hydrogens attached to the other nitrogen are replaced by a methyl group and a 6-{[4-(4-ethylpiperazin-1-yl)phenyl]amino}pyrimidin-4-yl group. It is a potent and selective fibroblast growth factor receptor inhibitor. It has a role as a fibroblast growth factor receptor antagonist and an antineoplastic agent. It is an aminopyrimidine, a N-arylpiperazine, a N-alkylpiperazine, a dichlorobenzene and a member of phenylureas.
brand name
Truseltiq
General Description
Class: receptor tyrosine kinase; Treatment: cholangiocarcinoma; Other name: NVP-BGJ398; Elimination half-life = 34 h; Protein binding = 96.8%
Pharmacokinetics
Infigratinib is an anti-tumour agent that works to suppress tumour growth in cholangiocarcinoma. It exhibits anti-tumour activity in mouse and rat xenograft models of human tumours with activating FGFR2 or FGFR3 alterations, such as FGFR2-TTC28 or FGFR2-TRA2B fusions. In clinical trials, patients with cholangiocarcinoma who were treated with infigratinib had an overall response rate of 23% - where one patient had a complete response - and a duration of response of 5.5 months, with a range between 0.03 and 28.3 months. Some patients with cancers with FGFR mutations display intrinsic resistance to infigratinib, leading to negligible therapeutic efficacy: investigations are ongoing to target molecular pathways to combat drug resistance.
Metabolism
According to in vitro findings, about 94% of infigratinib is metabolized by CYP3A4 and about 6% of the drug is metabolized by flavin-containing monooxygenase 3 (FMO3). About 38% of the dose is circulating parent drug in the plasma and BHS697 and CQM157 are two major metabolites of infigratinib that are each found at >10% of the dose. They are pharmacologically active, with BHS697 representing about 16% to 33% of the overall pharmacological activity of infigratinib and CQM157 contributing to about 9% to 12%. BHS697 undergoes further metabolism mediated by CYP3A4 and CQM157 is metabolized through both Phase I and Phase II biotransformation pathways. The exact metabolic pathways and the structure of BHS697 and CQM157 are not fully characterized.
References
1) Guagnano?et al.?(2011),?Discovery of 3-(2,6-Dichloro-3,5-dimethoxyphenyl)-1-(6-{[4-(4-ethyl-1-piperazinyl)phenyl]amino}-4-pyrimidinyl)-1-methylurea (NVP-BGJ398),?a Potent and Selective Inhibitor of the Fibroblast Growth Factor Receptor Family of Receptor Tyrosine Kinase?; J. Med. Chem.?54?7066 2) Komla-Ebri?et al.?(2016),?Tyrosine kinase inhibitor NVP-BGJ398 functionally improves FGFR3-related dwarfism in mouse model; J. Clin. Invest.?126?1871
Properties of Infigratinib
| Melting point: | >211°C (dec.) |
| Boiling point: | 747.9±60.0 °C(Predicted) |
| Density | 1.354 |
| storage temp. | -20°C |
| solubility | Soluble in DMSO (up to 5 mg/ml) |
| form | White solid. |
| pka | 11.02±0.70(Predicted) |
| color | White |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 2 months. |
Safety information for Infigratinib
Computed Descriptors for Infigratinib
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 NVP-BGJ398 >95% CAS 872511-34-7View Details
NVP-BGJ398 >95% CAS 872511-34-7View Details
872511-34-7 -
 Nvp-bgj398 95% CAS 872511-34-7View Details
Nvp-bgj398 95% CAS 872511-34-7View Details
872511-34-7 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1