Neratinib
- CAS NO.:698387-09-6
- Empirical Formula: C30H29ClN6O3
- Molecular Weight: 557.04
- MDL number: MFCD09752958
- EINECS: 811-237-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18
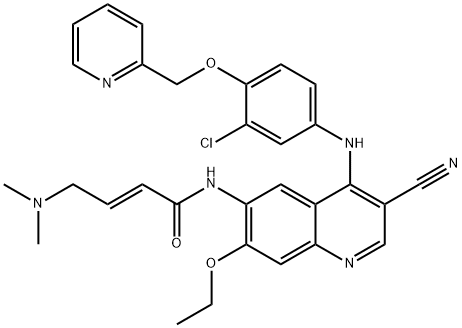
What is Neratinib?
Absorption
Neratinib and its major active metabolites M3. M6, and M7 have a Tmax of 2-8 h [FDA Label]. Administration with a high fat meal increases Cmax by 1.7-fold and total exposure by 2.2-fold. Administration with a standard meal increases Cmax by 1.2-fold and total exposure by 1.1-fold. Administration with gastric acid reducing agents such as proton pump inhibitors reduces Cmax by 71% and total exposure by 65%.
Toxicity
Use of neratinib may produce diarrhea and hepatotoxicity as clinically significant adverse effects [FDA Label]. Serious adverse reactions in the neratinib arm of the clinical trials included diarrhea (1.6%), vomiting (0.9%), dehydration (0.6%), cellulitis (0.4%), renal failure (0.4%), erysipelas (0.4%), alanine aminotransferase increase (0.3%), aspartate aminotransferase increase (0.3%), nausea (0.3%), fatigue (0.2%), and abdominal pain (0.2%).
Description
The receptor tyrosine kinase HER2 (ErbB2) is a key component of epidermal growth factor receptor complexes that are known to have central roles in cell proliferation and cancer. Neratinib is an orally active, irreversible inhibitor of the HER2 kinase (IC50 = 59 nM). It also potently inhibits several mutants of HER2 and shows 10-fold or greater selectivity over a wide variety of other kinases. As an irreversible inhibitor, neratinib may circumvent acquired resistance developed against reversible inhibitors, including gefitinib . Neratinib has been evaluated in clinical trials against cancers characterized by HER2 overexpression or HER2 activating mutations.
Characteristics
Class: receptor tyrosine kinase
Treatment: HER2-positive breast cancer
Elimination half-life = 14.6 h
Protein binding = 99%
The Uses of Neratinib
Neratinib (HKI-272) is a highly selective HER2 and EGFR inhibitor with IC50 of 59 nM and 92 nM, respectively.
The Uses of Neratinib
An oral, irreversible dual EGFR/HER2 inhibitor for breast and non-small cell lung cancer. Antitumor agent.
Background
Neratinib was approved in July 2017 for use as an extended adjuvant therapy in Human Epidermal Growth Factor Receptor 2 (HER2) positive breast cancer. Approval was granted to Puma Biotechnology Inc. for the tradename Nerlynx. Neratinib is currently under investigation for use in many other forms of cancer.
Indications
For use as an extended adjuvant treatment in adult patients with early stage HER2-overexpressed/amplified breast cancer, to follow adjuvant trastuzumab-based therapy [FDA Label].
What are the applications of Application
Neratinib is a potent and selective inhibitor of EGFR and HER2
Definition
ChEBI: A quinoline compound having a cyano group at the 3-position, a 3-chloro-4-(2-pyridylmethoxy)anilino group at the 4-position, a 4-dimethylamino-trans-but-2-enamido group at the 6-position, and an ethoxy group at the 7-position.
Pharmacokinetics
Neratinib is a tyrosine kinase inhibitor which exhibits antitumor action against Epidermal Growth Factor Receptor (EGFR), HER2, and Human Epidermal Growth Factor Receptor 4 (HER4) postive carcinomas [FDA Label].
Metabolism
Neratinib is mainly undergoes metabolism via CYP3A4 [FDA Label]. It is also metabolized by flavin-containing monooxygenase to a lesser extent. The systemic exposures of neratinib's active metabolites M3, M6, M7, and M11 are 15%, 33%, 22%, and 4%.
Storage
Store at -20°C
Properties of Neratinib
| Melting point: | 185-187°C |
| Boiling point: | 757.0±60.0 °C(Predicted) |
| Density | 1.33 |
| storage temp. | Refrigerator |
| solubility | Chloroform (Slightly), DMSO (Slightly, Heated, Sonicated), Methanol (Slightly, Heated) |
| form | Off-white solid. |
| pka | 12.37±0.43(Predicted) |
| color | Pale Yellow to Yellow |
Safety information for Neratinib
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H290:Corrosive to Metals H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P234:Keep only in original container. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P363:Wash contaminated clothing before reuse. P390:Absorb spillage to prevent material damage. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P405:Store locked up. P406:Store in corrosive resistant/… container with a resistant inner liner. |
Computed Descriptors for Neratinib
Neratinib manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Neratinib >95% CAS 698387-09-6View Details
Neratinib >95% CAS 698387-09-6View Details
698387-09-6 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8