NICKEL TETRAFLUOROBORATE
- CAS NO.:14708-14-6
- Empirical Formula: BF4Ni+
- Molecular Weight: 145.5
- MDL number: MFCD00016249
- EINECS: 238-753-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
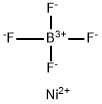
What is NICKEL TETRAFLUOROBORATE?
General Description
Green odorless aqueous solution. Sinks in and mixes with water.
Air & Water Reactions
Water soluble.
Reactivity Profile
Acidic inorganic salts, such as NICKEL TETRAFLUOROBORATE, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.
Health Hazard
Ingestion causes irritation of mouth and stomach. Liquid irritates eyes and skin and may cause dermatitis.
Safety Profile
ConfKmed carcinogen. Moderately toxic by ingestion and inhalation. When heated to decomposition it emits very toxic fumes of F-. See also FLUORIDES, BORON, and NICKEL COMPOUNDS.
Properties of NICKEL TETRAFLUOROBORATE
| Density | 1.47 |
| CAS DataBase Reference | 14708-14-6(CAS DataBase Reference) |
| EPA Substance Registry System | Borate(1-), tetrafluoro-, nickel(2+) (2:1) (14708-14-6) |
Safety information for NICKEL TETRAFLUOROBORATE
Computed Descriptors for NICKEL TETRAFLUOROBORATE
NICKEL TETRAFLUOROBORATE manufacturer
Madras Fluorine Private Ltd
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

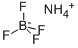
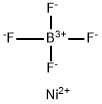
You may like
-
 Nickel tetrafluoroborate 98%View Details
Nickel tetrafluoroborate 98%View Details -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 207557-35-5 99%View Details
207557-35-5 99%View Details
207557-35-5 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1