Ammonium fluoborate
Synonym(s):Ammonium fluoroborate;Ammonium tetrafluoroborate
- CAS NO.:13826-83-0
- Empirical Formula: BF4H4N
- Molecular Weight: 104.84
- MDL number: MFCD06245785
- EINECS: 237-531-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
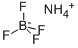
What is Ammonium fluoborate?
Chemical properties
white crystalline powder
The Uses of Ammonium fluoborate
Ammonium fluoroborate is used as an analytical reagent. It is also applied in the textile printing and dyeing industry and used as a catalyst for resin finishing. It is mainly used for the high temperature fluxing action required by the metals industry; catalyst; in flame retardants and acts as a solid lubricant in cutting-oil emulsions for aluminum rolling and forming.
General Description
Odorless white crystals. Sinks and mixes with water.
Air & Water Reactions
Sublimes above 238°C. to form toxic fumes [AAR 1991]. Water soluble.
Reactivity Profile
Acidic inorganic salts are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.
Health Hazard
INHALATION: May cause irritation of respiratory passages, nose bleeds, and nausea. EYES: May irritate.
Fire Hazard
Behavior in Fire: Sublimes above 238°C yielding toxic fumes.
Safety Profile
A poison and strong irritant. See also FLUORIDES and BORON COMPOUNDS. When heated to decomposition it emits very toxic fumes of F-, NO,, and NH3.
Purification Methods
Crystallise it from conductivity water (1mL/g) between 100o and 0o. 450 Purification of Inorganic Compounds
Properties of Ammonium fluoborate
| Melting point: | 230 °C |
| Density | 1.871 g/mL at 25 °C(lit.) |
| storage temp. | Inert atmosphere,Room Temperature |
| form | Powder |
| color | White |
| Specific Gravity | 1.871 |
| PH | 3.5 (5g/l, H2O, 20℃) |
| Water Solubility | soluble |
| Sensitive | Moisture Sensitive |
| Exposure limits | ACGIH: TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3; TWA 2.5 mg/m3 |
| CAS DataBase Reference | 13826-83-0(CAS DataBase Reference) |
| EPA Substance Registry System | Ammonium fluoroborate (13826-83-0) |
Safety information for Ammonium fluoborate
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Ammonium fluoborate
Ammonium fluoborate manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

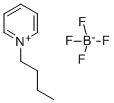
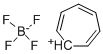
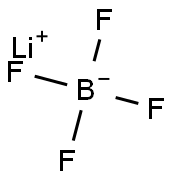
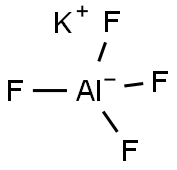
You may like
-
 AMMONIUM FLUOBORATE 99%View Details
AMMONIUM FLUOBORATE 99%View Details -
 Ammonium tetrafluoroborate 98%View Details
Ammonium tetrafluoroborate 98%View Details -
 Ammonium tetrafluoroborate CAS 13826-83-0View Details
Ammonium tetrafluoroborate CAS 13826-83-0View Details
13826-83-0 -
 Ammonium tetrafluoroborate CAS 13826-83-0View Details
Ammonium tetrafluoroborate CAS 13826-83-0View Details
13826-83-0 -
 Ammonium tetrafluoroborate CAS 13826-83-0View Details
Ammonium tetrafluoroborate CAS 13826-83-0View Details
13826-83-0 -
 Ammonium tetrafluoroborate CAS 13826-83-0View Details
Ammonium tetrafluoroborate CAS 13826-83-0View Details
13826-83-0 -
 Ammonium tetrafluoroborate CAS 13826-83-0View Details
Ammonium tetrafluoroborate CAS 13826-83-0View Details
13826-83-0 -
 Ammonium tetrafluoroborate CAS 13826-83-0View Details
Ammonium tetrafluoroborate CAS 13826-83-0View Details
13826-83-0
