Methanesulfonic acid
Synonym(s):Methanesulfonic acid;Methanesulfonic acid solution;MSA
- CAS NO.:75-75-2
- Empirical Formula: CH4O3S
- Molecular Weight: 96.11
- MDL number: MFCD00007518
- EINECS: 200-898-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
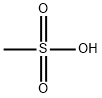
What is Methanesulfonic acid?
Description
Methanesulfonic acid (MSA), the simplest alkanesulfonic acid, is a hygroscopic colorless liquid or white solid, depending on whether the ambient temperature is greater or less than 20 oC. It is very soluble in water and oxygenated solvents, but sparingly soluble in most hydrocarbons. In aqueous solution, it is a strong acid (completely ionized).
MSA’s acidity and solubility properties make it industrially valuable as a catalyst in organic reactions, particularly polymerization. In many applications, its advantage over concentrated sulfuric acid is that it has similar acid strength but is not an oxidant.
The first report of MSA synthesis was in a 1950 patent awarded to John C. Snyder and Aristid V. Grosse of Houdry Process Corp. (subsequently acquired by Air Products). They heated methane and sulfur trioxide to 200–325 oC under pressure in the presence of a mercury catalyst. BASF currently produces the acid via a two-step process in which methanol and elemental sulfur react to give dimethyl disulfide, which is then oxidized to the final product.
For decades, chemists tried to find a way to prepare methanesulfonic acid from methane and sulfur trioxide under much milder conditions than were used in the original method. In 2015, Grillo-Werke (Duisburg, Germany), in a world patent application, described the preparation of alkanesulfonic acids from alkanes and SO3?in the presence of an organic peroxide at temperatures up to 65 oC and pressures up to 11 MPa. In June of this year, Grillo announced plans to compete with BASF by?building a plant to produce MSA?with a process that is likely based on that patent.
Grillo believes that its process will increase the supply of MSA so that it can be used for more applications. The company says that the plant should be operating by 2019
Chemical properties
Methanesulfonic acid is a colourless or light yellow liquid having a melting point of 20° C, is a strong acid acting corroding but not oxidizing.
Methanesulfonic acid is used in the electroplating industry and for organic syntheses, in particular as a catalyst for alkylations, esterifications, and polymerizations. Beyond that, methanesulfonic acid is used as a starting material for the preparation of methanesulfonyl chloride.
The Uses of Methanesulfonic acid
Polymerization catalyst.
Methanesulfonic acid has been developed as an esterification catalyst in place of sulfuric acid for the synthesis of resins in paints and coatings. One of the major advantages of methanesulfonic acid over sulfuric acid is that it is not an oxidizing species.
The Uses of Methanesulfonic acid
Methanesulfonic acid is used as a catalyst in organic reactions namely esterification, alkylation and condensation reactions due to its non- volatile nature and solubility in organic solvents. It is also involved in the production of starch esters, wax oxidate esters, benzoic acid esters, phenolic esters, or alkyl esters. It reacts with sodium borohydride in presence of polar solvent tetrahydrofuran to prepare borane-tetrahydrofuran complex. It finds application in batteries, because of its purity and chloride absence. In pharmaceutical industry, it is used for the manufacturing of active pharmaceutical ingredients like telmisartan and eprosartan. It is useful in ion chromatography and is a source of carbon and energy for some gram-negative methylotropic bacteria.It is involved in the deprotection of peptides.
The Uses of Methanesulfonic acid
For complete protein and peptide hydrolysis with tryptophan recovery. After hydrolysis the samples are diluted prior to amino acid analysis.
What are the applications of Application
Methanesulfonic acid solution is useful for ion chromatography
Definition
ChEBI: An alkanesulfonic acid in which the alkyl group directly linked to the sulfo functionality is methyl.
Preparation
Methanesulfonic acid is produced predominantly by oxidizing methylthiol or dimethyl disulfide using nitric acid, hydrogen peroxide, chlorine or by employing electrochemical processes.
General Description
Methanesulfonic acid (MSA) is a strong organic acid. The chemical oxidation of dimetyl sulfide in the atmosphere leads to the formation of MSA in large quantities. MSA undergoes biodegradation by forming CO2 and sulphate. It is considered a green acid as it is less toxic and corrosive in comparison to mineral acids.1 The aqueous MSA solution has been considered a model electrolyte for electrochemical processes.
Hazard
Toxic by ingestion, skin irritant, corrosiveto tissue.
Safety Profile
Poison by ingestion and intraperitoneal routes. May be corrosive to skin, eyes, and mucous membranes. Explosive reaction with ethyl vinyl ether. Incompatible with hydrogen fluoride. When heated to decomposition it emits toxic fumes of SOx. See also SULFONATES.
Purification Methods
Dry the acid, either by azeotropic removal of water with *benzene or toluene, or by stirring 20g of P2O5 with 500mL of the acid at 100o for 0.5hours. Then distil it under vacuum and fractionally crystallise it by partial freezing. Sulfuric acid, if present, can be removed by prior addition of Ba(OH)2 to a dilute solution, filtering off the BaSO4 and concentrating under reduced pressure; and is sufficiently pure for most applications. [Beilstein 4 IV 10.]
Properties of Methanesulfonic acid
| Melting point: | 17-19 °C (lit.) |
| Boiling point: | 167 °C/10 mmHg (lit.) |
| Density | 1.475-1.485 g/mL at 20 °C
1.481 g/mL at 25 °C (lit.) |
| vapor density | 3.3 (vs air) |
| vapor pressure | 1 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | 2-8°C |
| solubility | water: soluble1,000 g/L at 20°C |
| form | Solution |
| pka | -2.6(at 25℃) |
| Specific Gravity | 1.48 (18/4℃) |
| color | brown |
| Water Solubility | Miscible with water. Slightly miscible with benzene and toluene. Immiscible with paraffins. |
| Sensitive | Light Sensitive & Hygroscopic |
| λmax | λ: 240-320 nm Amax: <0.4 |
| Merck | 14,5954 |
| BRN | 1446024 |
| Stability: | Stable. Moisture sensitive. Incompatible with amines, bases, water, common metals. Releases a substantial amount of heat when diluted with water (add acid to water with care if diluting). |
| CAS DataBase Reference | 75-75-2(CAS DataBase Reference) |
| NIST Chemistry Reference | CH3SO3H(75-75-2) |
| EPA Substance Registry System | Methanesulfonic acid (75-75-2) |
Safety information for Methanesulfonic acid
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H290:Corrosive to Metals H302:Acute toxicity,oral H314:Skin corrosion/irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P234:Keep only in original container. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Methanesulfonic acid
| InChIKey | AFVFQIVMOAPDHO-UHFFFAOYSA-N |
Methanesulfonic acid manufacturer
Glindia Chemicals
ARRAKIS INDUSTRIES LLP
Anand Agencies
ASM Organics
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran





You may like
-
 Methanesulfonic Acid CAS 75-75-2View Details
Methanesulfonic Acid CAS 75-75-2View Details
75-75-2 -
 Methanesulfonic acid, 70% Solution in Water CAS 75-75-2View Details
Methanesulfonic acid, 70% Solution in Water CAS 75-75-2View Details
75-75-2 -
 Methane Sulfonic Acid (SQ) CAS 75-75-2View Details
Methane Sulfonic Acid (SQ) CAS 75-75-2View Details
75-75-2 -
 Methanesulphonic Acid (MSA) pure CAS 75-75-2View Details
Methanesulphonic Acid (MSA) pure CAS 75-75-2View Details
75-75-2 -
 Methanesulfonic acid 98% CAS 75-75-2View Details
Methanesulfonic acid 98% CAS 75-75-2View Details
75-75-2 -
 Methanesulfonic acid CAS 75-75-2View Details
Methanesulfonic acid CAS 75-75-2View Details
75-75-2 -
 Methanesulfonic Acid CAS 75-75-2View Details
Methanesulfonic Acid CAS 75-75-2View Details
75-75-2 -
 METHANE SULFONIC ACID CAS 75-75-2View Details
METHANE SULFONIC ACID CAS 75-75-2View Details
75-75-2