Maslinic acid
Synonym(s):2α,3β-Dihydroxyolean-12-en-28-oic acid;2α-Hydroxyoleanolic acid;Crategolic acid
- CAS NO.:4373-41-5
- Empirical Formula: C30H48O4
- Molecular Weight: 472.7
- MDL number: MFCD00049293
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 12:07:08
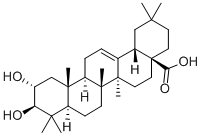
What is Maslinic acid?
Description
Maslinic acid (2-α,3-β-dihydroxyolean-12-en-28-oic acid) is a pentacyclic triterpene abundant in the cuticular lipid layer of olive fruits (Bianchi et al.,1994) .It is a compound of 30 carbon atoms grouped in five cycles that have several substitutes. Maslinic acid presents two hydroxyl groups bound to carbons 2 and 3,one carboxyl group bound to carbon 17, and a double bond between carbons 12 and 13.It is a highly hydrophobic compound of low water solubility. Maslinic acid is synthesized in plants via the cytoplasmic acetate/mevalonate pathway that leads to oxidosqualene (Seo et al., 1988).Oxidosqualene is cycled by various oxidosqualene cyclases, among them β-amyrin synthase, which catalyzes the transformation of oxydosqualene into β-amyrin (olean-12-3n-3β-ol). Afterwards,β-amyrin is converted by successive reactions into erythrodiol, oleanolic acid, and finally maslinic acid (Saimaru et al.,2007; Stiti et al.,2007).
Description
Maslinic acid is found in a number of natural sources, most notably pomace olive oil (orujo). This pentacyclic triterpene has an antiproliferative effect against Caco-
The Uses of Maslinic acid
Maslinic Acid is an apoptosis inducer in lung cancer cells, occuring under normoxic and hypoxic conditions.
The Uses of Maslinic acid
Maslinic acid may be used as an analytical reference standard for the quantification of the analyte in the leaves of Ziziphus species and fruits using different chromatography techniques.
What are the applications of Application
Maslinic Acid is a pentacyclic triterpene with antiproliferative properties
Definition
Maslinic acid is a pentacyclic triterpenoid that is olean-12-ene substituted by hydroxy groups at positions 2 and 3 and a carboxy group at position 28 (the 2alpha,3beta stereoisomer). It is isolated from Olea europaea and Salvia canariensis and exhibits anti-inflammatory, antioxidant and antineoplastic activity. It has a role as an antioxidant, an antineoplastic agent, an anti-inflammatory agent and a plant metabolite. It is a pentacyclic triterpenoid and a dihydroxy monocarboxylic acid. It derives from a hydride of an oleanane.
General Description
Maslinic acid belongs to the class of triterpenic acids, a group of phytochemicals present in a large variety of plants. It exhibits a broad-spectrum of biological properties such as cardiovascular, antihyperlipidemic, antioxidant effects, hepatoprotective effects, antitumor activity. It is also involved in enhancing the cellular immune system.
Biological Activity
Maslinic acid is found in a number of natural sources, most notably pomace olive oil (orujo). This pentacyclic triterpene has an antiproliferative effect against Caco-2 cancer cells (EC50 = 15 μM), HT-29 human colon cancer cells (EC50 = 74 μM), 1321N1 astrocytoma cells (IC50 = 25 μM), and human leukemia (CCRF-CEM and CEM/ADR5000) cells (IC50 = 7 μM and 9 μM respectively). Maslinic acid's antiproliferative activity likely comes from the induction of an oxidative apoptotic pathway, causing cell cycle and cytoskeleton alterations. Maslinic acid has been found to attenuate intracellular oxidative stress via inhibition of NO and H2O2 production and reduction of pro-inflammatory cytokine generation in murine macrophages. Recently maslinic acid has been found to inhibit the spread of the HIV virus by inhibiting the replication of a primary HIV-1 isolate as well as decreased the cytopathic effect and p24 antigen levels in MT2 cells.
Biochem/physiol Actions
Predominant triterpenoid found in olives. Anti-proliferative. Promising chemopreventive agent.
Cytotoxicity
IC50 (μg/mL): 6.48 (518A2), 13.61(HT29),17.58 (MCF-7), 11.06 (A549), 9.22(A2780), 8.04 (8505C) (Siewert et al.2014)
IC50 (μg/mL): 9.97 (NIH-3T3)(Sommerwerk et al. 2016).
References
[1] reyes-zurita f j, rufino-palomares e e, lupiáez j a, et al. maslinic acid, a natural triterpene from olea europaea l., induces apoptosis in ht29 human colon-cancer cells via the mitochondrial apoptotic pathway[j]. cancer letters, 2009, 273(1): 44-54.
[2] garcía, a. compound from olive-pomace oil inhibits hiv spread. 070709111536, 1-1 (2007).
[3] liu j, sun h, duan w, et al. maslinic acid reduces blood glucose in kk-ay mice[j]. biological and pharmaceutical bulletin, 2007, 30(11): 2075-2078.
[4] guan t, qian y, tang x, et al. maslinic acid, a natural inhibitor of glycogen phosphorylase, reduces cerebral ischemic injury in hyperglycemic rats by glt‐1 up‐regulation[j]. journal of neuroscience research, 2011, 89(11): 1829-1839.
Properties of Maslinic acid
| Melting point: | 267~269℃ |
| Boiling point: | 570.0±50.0 °C(Predicted) |
| Density | 1.14±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | acetone: soluble1mg/mL, clear, colorless |
| pka | 4.63±0.70(Predicted) |
| form | powder |
| color | white to off-white |
| Stability: | Hygroscopic |
Safety information for Maslinic acid
Computed Descriptors for Maslinic acid
| InChIKey | MDZKJHQSJHYOHJ-LLICELPBSA-N |
| SMILES | [C@@]12(C)CC[C@@]3([H])C(C)(C)[C@@H](O)[C@H](O)C[C@]3(C)[C@@]1([H])CC=C1[C@]3([H])CC(CC[C@]3(C(=O)O)CC[C@@]21C)(C)C |&1:0,4,9,11,14,16,21,27,33,r| |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

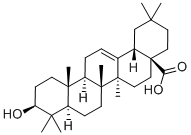


![1,7-DIOXASPIRO[5.5]UNDECANE](https://img.chemicalbook.in/CAS/GIF/180-84-7.gif)
You may like
-
 Maslinic acid CAS 4373-41-5View Details
Maslinic acid CAS 4373-41-5View Details
4373-41-5 -
 Maslinic acid CAS 4373-41-5View Details
Maslinic acid CAS 4373-41-5View Details
4373-41-5 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
