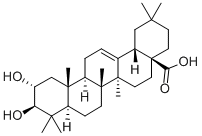
Oleanolic acid
- CAS NO.:508-02-1
- Empirical Formula: C30H48O3
- Molecular Weight: 456.7
- MDL number: MFCD00064914
- EINECS: 208-081-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 08:49:36
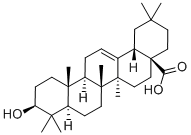
What is Oleanolic acid?
Description
Oleanolic acid or oleanic acid is a naturally occurring pentacyclic triterpenoid with the formula of C30H48O3. It was found in the non-glyceride fraction of olive pomace oil and is widely distributed in food and plants where it exists as a free acid or as an aglycone precursor for triterpenoid saponins, in which it can be linked to one or more sugar chains. It is biosynthesized from lupane. It can rearrange to the isomer, ursolic acid, or be oxidized to taraxasterol and amyrin. Oleanolic acid and its derivatives possess several promising pharmacological activities, such as hepatoprotective effects, and anti-inflammatory, antioxidant, or anticancer activities.
Chemical properties
White needle-like crystals (ethanol), odorless, tasteless. Unstable to acid and alkali. Insoluble in water, soluble in methanol, ethanol, ethyl ether, acetone and chloroform.
Occurrence
The ingredient is mainly extracted from the leaves of Olea europaea and Viscum album L. Besides, it is found in the fruit of ligustrum lucidum ait, the whole grass of swertia mileensis t.n.he et w.l.shi, s. mussotii franch, the leaves and roots of astrantia major, the root bark and stem bark of aralia chinensis, the roots of hemsleya macrosperma c.y.wu , the roots of hemsleya amabilis diels, and the roots of hemsleya chinensis cogn. There is a long history in the herb and fruit which contain oleanolic acid. They play an important role in the treatment of various diseases. Oleanolic acid may be the most effective component in various Chinese medicines.
History
The chemical formula was first identified in 1918. In 1960, Khastgir purified the compound for the first time. Corey identified the structure of oleanolic acid in 1993.Now the compound has been made into a drug and is being marketed in China. As early as 2002, the tablet form of oleanolic acid was listed. In 2010, capsule preparations came into the market, and to date, there is no other listed preparation. Sanofi treated it as antiulcer and NSAIDs candidate in abroad.Although it has anti-inflammatory and anti-ulcer effects, Sanofi stopped the study of developing oleanolic acid into a drug in 1989.
The Uses of Oleanolic acid
Oleanolic acid is a triterpenoid known for its anti-inflammatory properties, is commonly present in several medicinal plants. It can Inhibits secretory phospholipase A2 that is mainly used for the treatment of acute jaundiced hepatitis with remarkable efficacy, and also has good effect on chronic hepatitis.?
What are the applications of Application
Oleanolic Acid is a compound useful for the induction of iNOS and of Cyclooxygenase 2
Definition
ChEBI: Oleanolic acid is a pentacyclic triterpenoid that is olean-12-en-28-oic acid substituted by a beta-hydroxy group at position 3. It has a role as a plant metabolite. It is a pentacyclic triterpenoid and a hydroxy monocarboxylic acid. It is a conjugate acid of an oleanolate. It derives from a hydride of an oleanane.
Indications
Oleanolic acid is included in the Pharmacopoeia of the People's Republic of China (Part 2, volume 3), the British Pharmacopoeia (2017), and the European Pharmacopoeia (8.7th ed.). It is mainly used for the adjuvant treatment of acute infectious icteric hepatitis and acute and chronic hepatitis. It also can be utilized in psoriasis, rheumatoid arthritis, nephritic edema, ascites, stomach with turbid, metrorrhagia, bruises, carbuncle, soreness and weakness of the waist and knees, fetal irritability and so on.
General Description
Oleanolic acid is a hydroxyl pentacyclic triterpenoic acid (HPTA), which exhibits antibacterial, antitumor and antileishmanial activity. It is biosynthesized from lupane. It can rearrange to the isomer, ursolic acid, or be oxidized to taraxasterol and amyrin.
Biochem/physiol Actions
Triterpenoid isolated from medicinal plants. Antitumor and hepatoprotective agent. Oleanolic acid has therapeutic potential for neurodegenerative diseases.
Pharmacology
Liver protection: Pretreatment of 100?mg/kg once a day for 3?days can protect the liver and decrease the activity of enzymes. It achieves by increasing the amount of metallothioneins and liver glucoside transferases to prevent glutathione emptying, to inhibit several subtypes of P450 oxidase, and to prevent combination with toxic substances.Antimicrobial and antiviral effect: Oleanolic acid may inhibit the proliferation or production of NO induced by interferon or in the macrophages by competitively binding to the enzymes necessary for the growth and reproduction of microorganisms or virus and play an anti-inflammatory and antiviral role. EC50 of HIV-1- infected H9 cells is 3.4?mol/L.Effects on metabolism of glucose and lipid: The treatment of 50–100?mg/kg for 4?days achieves the glucosidase activity inhibition, reduction of glucose absorption, inhibition of glycogen phosphorylase, activation of glycogen synthesis, inhibition of protein tyrosine phosphatase 1B, regulation of insulin signaling pathway, and increase of insulin sensitivity, and it may also be achieved by inhibiting the transport of glucose from the stomach to the intestine and transport activity of intestinal villi. 200?mg/kg suspension administrated by gavage for 4?weeks decreases the level of triglycerides, cholesterol, and β-lipoprotein in high-fat animal, and it contributes to increased platelet swimming rate and decreased blood viscosityEffects on immunologic function: 60?mg/kg intraperitoneal injection may regulate the immune system by inhibiting type I allergic reaction, promoting proliferation of lymphocyte and inhibiting enzyme C-3? in the traditional complement pathway.Antioxidant effect: It fights against lipid peroxidation by scavenging free radicals.Other functions: 10–5?mol/L oleanolic acid may inhibit the activity of DNA ligase I active site. It exerts anticancer and anti-mutation effects, and IC50 value is2.2×105 ?mol/L in inhibiting ornithine decarboxylase (ODC) induced by external TPA at the transcriptional level. Oleanolic acid in polyvinylpyrrolidone suspension at the dose of 16?mg/kg by intragastric administration for 30?days also reversibly decreases quality and exercise capacity of mouse sperms.It is reported that it is consistent with the single compartment model, or noncompartmental model, and may be distributed in the compound in accordance with the two compartment models. Eventually, it will be eliminated by P450 oxidase in the liver.
Clinical Use
It is reported that contrast with Western medicine, the 60~90?mg/d oleanolic acid tablets supplemented with vitamin B can treat acute and chronic hepatitis, with effective rates of 94.4% and 69.81%, respectively, in clinical results. It has been listed on the drug manual as adjuvant therapy for acute and chronic hepatitis.
References
1.https://en.wikipedia.org/wirki/Oleanolic_acid
2.https://www.ncbi.nlm.nih.gov/mesh/68009828
3.http://www.sciencedirect.com/science/article/pii/S0031942212000027
4.http://www.adooq.com/oleanolic-acid-caryophyllin.html
Properties of Oleanolic acid
| Melting point: | >300 °C (lit.) |
| Boiling point: | 502.79°C (rough estimate) |
| alpha | D20 +83.3° (c = 0.6 in chloroform) |
| Density | 1.0261 (rough estimate) |
| refractive index | 1.4940 (estimate) |
| storage temp. | 2-8°C |
| solubility | Chloroform (Very Slightly), DMSO (Slightly, Heated), Methanol (Slightly, Heated) |
| form | neat |
| form | Solid |
| pka | 2.52(at 25℃) |
| color | light yellow |
| Merck | 14,6827 |
| CAS DataBase Reference | 508-02-1(CAS DataBase Reference) |
| EPA Substance Registry System | Oleanolic acid (508-02-1) |
Safety information for Oleanolic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Oleanolic acid
| InChIKey | NZQIXFRGWXNLSP-IDYUENATSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Oleanolic acid, 99% CAS 508-02-1View Details
Oleanolic acid, 99% CAS 508-02-1View Details
508-02-1 -
 Oleanolic acid hydrate 96% CAS 508-02-1View Details
Oleanolic acid hydrate 96% CAS 508-02-1View Details
508-02-1 -
 Oleanolic acid dihydrate 95% CAS 508-02-1View Details
Oleanolic acid dihydrate 95% CAS 508-02-1View Details
508-02-1 -
 Oleanolic acid CAS 508-02-1View Details
Oleanolic acid CAS 508-02-1View Details
508-02-1 -
 Oleanolic Acid CAS 508-02-1View Details
Oleanolic Acid CAS 508-02-1View Details
508-02-1 -
 Oleanolic acid CAS 508-02-1View Details
Oleanolic acid CAS 508-02-1View Details
508-02-1 -
 Oleanolic acid CAS 508-02-1View Details
Oleanolic acid CAS 508-02-1View Details
508-02-1 -
 Oleanolic acid CAS 508-02-1View Details
Oleanolic acid CAS 508-02-1View Details
508-02-1
