LORCASERIN
- CAS NO.:616202-92-7
- Empirical Formula: C11H14ClN
- Molecular Weight: 195.69
- MDL number: MFCD09833670
- EINECS: 809-254-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-09-07 19:13:26
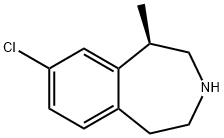
What is LORCASERIN?
Absorption
Lorcaserin has a peak plasma concentration of about 1.5 - 2 hours, but the bioavailability was not determined.
Toxicity
Most common adverse reactions include hypoglycemia (diabetic patients), headache, back pain,fatigue, decrease in lymphocytes,upper respiratory tract infection, and nasopharyngitis. Moreover, the safety and efficacy of coadministration with other weight loss products has not been established, and cardiovascular effects on mortality and morbidity have not been established.
Chemical properties
Yellow Oil
The Uses of LORCASERIN
Lorcaserin was used to treat Opioid Use Disorder (OUD).
The Uses of LORCASERIN
A novel selective 5-HT2C-receptor agonist for the treatment of obesity.
Indications
For the treatment of obesity, as an adjunct to a reduced-calorie diet and increased physical activity.
Background
Lorcaserin (previously APD-356), a highly selective 5HT2C receptor agonist, is used for the treatment of obesity. It has been shown to reduce body weight and food intake in animal models of obesity, and it is thought that targeting the 5HT2C receptor may alter body weight by regulating satiety. Lorcaserin is marketed as a salt form called Belviq, which is lorcaserin hydrochloride.
In February 2020, the FDA issued a Drug Safety Communication requesting the manufacturer of Belviq (lorcaserin hydrochloride tablets, 10 mg) and Belviq XR (lorcaserin hydrochloride extended-release tablets, 20 mg) to voluntarily withdraw these products from the U.S. market, and the company has submitted a request to voluntarily withdraw the drug. This decision was based on the results of a clinical trial assessing the risk of heart-related problems that found that patients treated with lorcaserin may have a higher risk of cancer.
Definition
ChEBI: A benzazepine that is 2,3,4,5-tetrahydro-3-benzazepine substituted at position 1 by a methyl group and a t position 6 by a chloro group.
Mechanism of action
Lorcaserin acts at 5-HT2C receptors in the central nervous system (CNS), particularly the hypothalamus, to reduce appetite.
Pharmacokinetics
Lorcaserin produced a dose-dependent weight loss over a 12-week period by promoting satiety and decreasing food consumption.
Side Effects
- constipation
- dry mouth
- excessive tiredness
- pain in the back or muscles
- headache
- dizziness
- difficulty falling asleep or staying asleep
- anxiety
Synthesis
The synthesis of LORCASERIN is as follows:
Liquid precursor 53 11-HCl (0.5mmol) was placed into a test tube equipped with a magnetic stir bar. Anhydrous 44 AlCl3 (1.75 equiv according to starting material) was added and efficiently mixed to obtain a paste. The mixture was slowly heated (10°C/min) to 150°C and stirred overnight. A saturated solution of 54 NaCl was added and the mixture was cooled. The pH was adjusted to 9.5-10 using 1M NaOH and then extracted with EtOAc. The combined organic phases were washed with brine, dried over Na2SO4 and the solvent was evaporated under reduced pressure. The obtained crude mixture was analyzed using 1H NMR spectroscopy (estimated yield by 1H NMR was 62%).
Metabolism
Lorcaserin has extensive hepatic metabolism producing inactive compounds. Lorcaserin sulfamate (M1) is the major metabolite circulating in the plasma, and N-carbamoyl glucuronide lorcaserin (M5) is the major metabolite in urine. Other minor metabolites that are both excreted in urine are glucuronide or sulfate conjugates.
Properties of LORCASERIN
| Boiling point: | 288 ºC |
| Density | 1.075 |
| Flash point: | 128 ºC |
| storage temp. | -20°C Freezer |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Light Beige to Beige Semi-Solid |
| pka | 9.99±0.40(Predicted) |
| Stability: | Hygroscopic |
| InChI | InChI=1S/C11H14ClN/c1-8-7-13-5-4-9-2-3-10(12)6-11(8)9/h2-3,6,8,13H,4-5,7H2,1H3/t8-/m0/s1 |
Safety information for LORCASERIN
Computed Descriptors for LORCASERIN
| InChIKey | XTTZERNUQAFMOF-QMMMGPOBSA-N |
| SMILES | N1CCC2=CC=C(Cl)C=C2[C@@H](C)C1 |
LORCASERIN manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
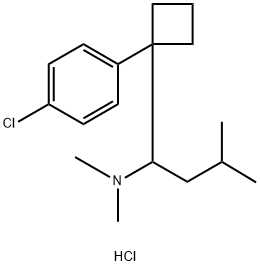
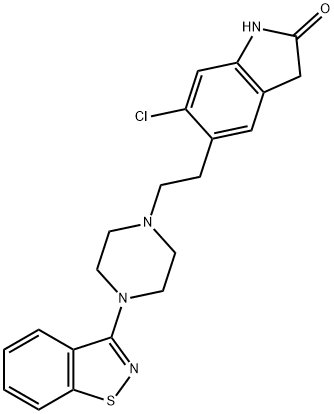
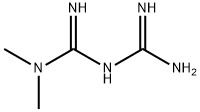
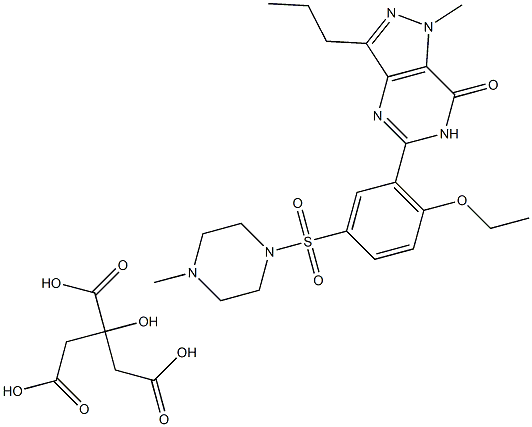
You may like
-
 616202-92-7 Lorcaserin 98%View Details
616202-92-7 Lorcaserin 98%View Details
616202-92-7 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8