Sibutramine
- CAS NO.:106650-56-0
- Empirical Formula: C17H26ClN
- Molecular Weight: 279.85
- MDL number: MFCD00866415
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-15 10:43:32
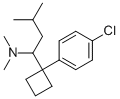
What is Sibutramine?
Absorption
Rapid absorption following oral administration. Absolute bioavailability is not known, but at least 77% of a single oral dose of sibutramine is absorbed.
Toxicity
Side effects include dry mouth, anorexia, insomnia, constipation and headache.
Originator
Meridia,Abbott Laboratories,USA
The Uses of Sibutramine
Anorexic; antidepressant.
Indications
For the treatment of obesity.
Background
Sibutramine (trade name Meridia in the USA, Reductil in Europe and other countries), usually as sibutramide hydrochloride monohydrate, is an orally administered agent for the treatment of obesity. It is a centrally acting stimulant chemically related to amphetamines thus it is classified as a Schedule IV controlled substance in the United States. In October 2010, Sibutramine was withdrawn from Canadian and U.S. markets due to concerns that the drug increases the risk of heart attack and stroke in patients with a history of heart disease.
Definition
ChEBI: A tertiary amino compound that is N,N,3-trimethylbutan-1-amine substituted by a (4-chlorophenyl)cyclobutyl group at position 1.
Manufacturing Process
A solution of 4-chlorobenzyl cyanide and 1,3-dibromopropane in dry
dimethylsulfoxide was added dropwise under nitrogen to a stirred mixture of
sodium hydride dispersed in mineral oil and dimethylsulfoxide at a
temperature in the range 30° to 35°C. The mixture was stirred at room
temperature for 2 h and propan-2-ol and then water were added dropwise.
The mixture was filtered through a diatomaceous earth sold under the
Registered Trade Mark CELITE and the solid residue washed with ether. The
ether layer was separated, washed with water, dried and evaporated. 1-(4-
Chlorophenyl)-1-cyclobutanecarbonitrile was isolated by distillation.
1-(4-Chlorophenyl)-1-cyclobutanecarbonitrile (37.6 g) was added to a solution
of potassium hydroxide (32.4 g) in diethyleneglycol (370 ml) and the mixture
heated under reflux for three and a 0.5 h The reaction mixture was poured
into an ice/water mixture and the resulting solution was washed with ether.
The aqueous layer was added to a mixture of concentrated hydrochloric acid
(100 ml) and ice and the resulting precipitate of 1-(4-chlorophenyl)-1-
cyclobutanecarboxylic acid (melting point 86°-88°C) collected, washed with
water and dried.
1-(4-Chlorophenyl)-1-cyclobutane carboxylic acid (10.5 g) was heated under
reflux with thionyl chloride (20 ml) for 2.5 h. Excess thionyl chloride was
evaporated off and the acid chloride of the above acid distilled (boiling point
82°-96°C /0.2 mm Hg). A solution of the acid chloride in dry tetrahydrofuran
was added slowly to the product of the reaction of magnesium turnings and
ethyl bromide in dry tetrahydrofuran. Water was added followed by 5 N
hydrochloric acid with cooling. The reaction mixture was extracted with ether,
washed with water, sodium bicarbonate solution, dried. The solvent was
removed by evaporation and 1-isobutyl-1-(4-chlorophenyl)cyclobutane
obtained by distillation. The 1-isobutyl-1-(4-chlorophenyl)cyclobutane,
diethylene glycoldimethyl ether, water and concentrated hydrochloric acid
were mixed and heated under reflux. The mixture was poured into water
aqueous NaOH was added and the product extracted into ether. Evaporation
gave a dark oil. A sample of this oil, water and formic acid were mixed and
formaldehyde added. The mixture was heated under reflux and then
concentrated hydrochloric acid and propan-2-ol were added. Evaporation to dryness gave N,N-dimethyl-1-[1-(4-chlorophenyl)cyclobutyl]isobutyl
hydrochloride as a white solid.
brand name
Meridia (Abbott).
Therapeutic Function
Antidepressant, Anorexic
General Description
Sibutramine (Meridia) is said to be an uptake inhibitor ofNE and 5-HT. These mechanisms fit its structure. It is reportedlyan antidepressant and an anorexiant drug. Thismechanism implies that activation of all presynaptic andpostsynaptic receptors in NE and 5-HT systems is possible.The data are not completely clear, but studies to date indicatethat the receptors principally involved are α1, β1, and5-HT2C.
Pharmacokinetics
Sibutramine is an orally administered agent for the treatment of obesity. Sibutramine exerts its pharmacological actions predominantly via its secondary (M1) and primary (M2) amine metabolites. The parent compound, sibutramine, is a potent inhibitor of serotonin and norepinephrine reuptake in vivo, but not in vitro. However, metabolites M1 and M2 inhibit the reuptake of these neurotransmitters both in vitro and in vivo. In human brain tissue, M1 and M2 also inhibit dopamine reuptake in vitro, but with ~3-fold lower potency than for the reuptake inhibition of serotonin or norepinephrine. Sibutramine, M1 and M2 exhibit no evidence of anticholinergic or antihistaminergic actions. In addition, receptor binding profiles show that sibutramine, M1 and M2 have low affinity for serotonin (5-HT1, 5-HT1A, 5-HT1B, 5-HT2A, 5-HT2C), norepinephrine (b, b1, b3, a1 and a2), dopamine (D1 and D2), benzodiazepine, and glutamate (NMDA) receptors. These compounds also lack monoamine oxidase inhibitory activity in vitro and in vivo.
Metabolism
Hepatic
Properties of Sibutramine
| Melting point: | 191-192°C |
| Boiling point: | 342.6±25.0 °C(Predicted) |
| Density | 1.031±0.06 g/cm3(Predicted) |
| solubility | Chloroform (Slightly), DMSO (Slightly), Ethyl Acetate (Slightly) |
| pka | 9.69±0.50(Predicted) |
| form | Solid |
| color | White to Off-White |
| Water Solubility | 2.9 mg/mL |
| CAS DataBase Reference | 106650-56-0(CAS DataBase Reference) |
Safety information for Sibutramine
Computed Descriptors for Sibutramine
Sibutramine manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Potassium Hydroxide 90%View Details
Potassium Hydroxide 90%View Details
1310-58-3 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
