Melatonine
Synonym(s):Melatonin;Melatonine;N-Acetyl-5-methoxytryptamine;N-Acetyl-5-methoxytryptamine, 5-Methoxy-N-acetyltryptamine;N-Acetyl-5-methoxytryptamine
- CAS NO.:73-31-4
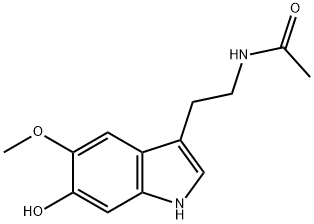
- Empirical Formula: C13H16N2O2
- Molecular Weight: 232.28
- MDL number: MFCD00005655
- EINECS: 200-797-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-03-15 17:35:32
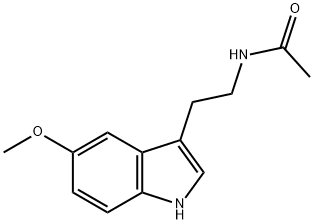
What is Melatonine?
Absorption
The absorption and bioavailability of melatonin varies widely.
Toxicity
Generally well-tolerated when taken orally. The most common side effects, day-time drowsiness, headache and dizziness, appear to occur at the same frequency as with placebo. Other reported side effects include transient depressive symptoms, mild tremor, mild anxiety, abdominal cramps, irritability, reduced alertness, confusion, nausea, vomiting, and hypotension. Safety in Adults: Evidence indicates that it is likely safe to use in oral and parenteral forms for up to two months when used appropriately. Some evidence indicates that it can be safely used orally for up to 9 months in some patients. It is also likely safe to use topically when used appropriately. Safety in Children: Melatonin appeared to be used safely in small numbers of children enrolled in short-term clinical trials. However, concerns regarding safety in children have arisen based on their developmental state. Compared to adults over 20 years of age, people under 20 produce high levels of melatonin. Melatonin levels are inversely related to gonadal development and it is thought that exogenous administration of melatonin may adversely affect gonadal development. Safety during Pregnancy: High doses of melatonin administered orally or parenterally may inhibit ovulation. Not advised for use in individuals who are pregnant or trying to become pregnant. Safety during Lactation: Not recommended as safety has not be established.
Oral, rat: LD50 ≥3200 mg/kg
Description
Melatonin, or N-acetyl-5-methoxytryptamine, is a hormone that is secreted by the pineal gland in mammals; it is also found in other animals, plants, and microorganisms. It helps regulate the circadian rhythms of biological functions. A. B. Lerner and co-workers isolated melatonin from cattle pineal glands in 1958 and characterized it a year later. The following year, J. Szmuszkovicz et al. published two laboratory syntheses of melatonin.
Description
Melatonin, at times referred to as the “hormone of darkness,” normally is secreted during the night. It is synthesized in the pineal gland, and its secretion is controlled by the suprachiasmatic nucleus, following an endogenous circadian rhythm. Studies indicate that melatonin may
Chemical properties
Off-White Powder
Occurrence
Melatonin is a naturally occurring hormone in the body.
The Uses of Melatonine
Melatonin is a neurohormone that upregulates antioxidant enzymes and inhibits rat NOS1. It can be used in sleep induction, modifies circadian rhythm, antioxidant, free radical scavenger.
The Uses of Melatonine
Hormone postulated to mediate photoperiodicity in mammals. Inhibits cerebellar nitric oxide synthetase
The Uses of Melatonine
Immunostimulant;Melatonin receptor ligand
The Uses of Melatonine
Melatonin has complex effects on apoptotic pathways, inhibiting apoptosis in immune cells and neurons but enhancing apoptotic cell death of cancer cells. Inhibits proliferation/metastasis of breast cancer cells by inhibiting estrogen receptor action.
Indications
Used orally for jet lag, insomnia, shift-work disorder, circadian rhythm disorders in the blind (evidence for efficacy), and benzodiazepine and nicotine withdrawal. Evidence indicates that melatonin is likely effective for treating circadian rhythm sleep disorders in blind children and adults. It has received FDA orphan drug status as an oral medication for this use. A number of studies have shown that melatonin may be effective for treating sleep-wake cycle disturbances in children and adolescents with mental retardation, autism, and other central nervous system disorders. It appears to decrease the time to fall asleep in children with developmental disabilities, such as cerebral palsy, autism, and mental retardation. It may also improve secondary insomnia associated with various sleep-wake cycle disturbances. Other possible uses for which there is some evidence for include: benzodiazepine withdrawal, cluster headache, delayed sleep phase syndrome (DSPS), primary insomnia, jet lag, nicotine withdrawal, preoperative anxiety and sedation, prostate cancer, solid tumors (when combined with IL-2 therapy in certain cancers), sunburn prevention (topical use), tardive dyskinesia, thrombocytopenia associated with cancer, chemotherapy and other disorders.
Background
Melatonin is a biogenic amine that is found in animals, plants and microbes. Aaron B. Lerner of Yale University is credited for naming the hormone and for defining its chemical structure in 1958. In mammals, melatonin is produced by the pineal gland. The pineal gland is small endocrine gland, about the size of a rice grain and shaped like a pine cone (hence the name), that is located in the center of the brain (rostro-dorsal to the superior colliculus) but outside the blood-brain barrier. The secretion of melatonin increases in darkness and decreases during exposure to light, thereby regulating the circadian rhythms of several biological functions, including the sleep-wake cycle. In particular, melatonin regulates the sleep-wake cycle by chemically causing drowsiness and lowering the body temperature. Melatonin is also implicated in the regulation of mood, learning and memory, immune activity, dreaming, fertility and reproduction. Melatonin is also an effective antioxidant. Most of the actions of melatonin are mediated through the binding and activation of melatonin receptors. Individuals with autism spectrum disorders (ASD) may have lower than normal levels of melatonin. A 2008 study found that unaffected parents of individuals with ASD also have lower melatonin levels, and that the deficits were associated with low activity of the ASMT gene, which encodes the last enzyme of melatonin synthesis. Reduced melatonin production has also been proposed as a likely factor in the significantly higher cancer rates in night workers.
Definition
ChEBI: A member of the class of acetamides that is acetamide in which one of the hydrogens attached to the nitrogen atom is replaced by a 2-(5-methoxy-1H-indol-3-yl)ethyl group. It is a hormone secreted by the pineal gland in humans.
World Health Organization (WHO)
Melatonin is promoted as a cure for travel sickness, jet-lag and insomnia and has recently been claimed in the United States to reverse the ageing process. A synthetic version has been freely available from health food shops and pharmacies as a "nutritional supplement" since 1993.
General Description
A hormone that has been postulated as being a mediator of photic-induced anti-gonadotropic activity in photoperiodic mammals. Counteracts the apoptotic effects of etoposide in bone marrow cells. Melatonin receptors are coupled to a G-protein system. Inhibits rat cerebellar nitric oxide synthase (NOS).
Biological Activity
Endogenous hormone that acts as an agonist at melatonin receptors MT 1 and MT 2 . Exhibits a prominent role in the control of circadian rhythm, displays immunomodulatory activity and acts as a powerful antioxidant in vivo .
Biochem/physiol Actions
Hormone; mediates photoperiodicity in mammals; inhibits cerebellar nitric oxide synthetase; peroxynitrite scavenger. Melatonin has complex effects on apoptotic pathways, inhibiting apoptosis in immune cells and neurons but enhancing apoptotic cell death of cancer cells. Inhibits proliferation/metastasis of breast cancer cells by inhibiting estrogen receptor action.
Pharmacokinetics
Melatonin is a hormone normally produced in the pineal gland and released into the blood. The essential amino acid L-tryptophan is a precursor in the synthesis of melatonin. It helps regulate sleep-wake cycles or the circadian rhythm. Production of melatonin is stimulated by darkness and inhibited by light. High levels of melatonin induce sleep and so consumption of the drug can be used to combat insomnia and jet lag. MT1 and MT2 receptors may be a target for the treatment of circadian and non circadian sleep disorders because of their differences in pharmacology and function within the SCN. SCN is responsible for maintaining the 24 hour cycle which regulates many different body functions ranging from sleep to immune functions
Clinical Use
Melatonin is most effective in young individuals and appears to be less effective in elderly individuals (possibly because of a decreased number of receptors). Melatonin causes a phase shift of approximately 1 hour per day. This means that the use of melatonin in the morning can delay the onset of evening sleepiness, whereas melatonin taken in the evening has been associated with faster onset of sleep and increased total sleep time. Melatonin is sold as a food supplement in the United States, but it has become popular for use as a hypnotic and for alleviating jet lag (a flight across five or more time zones) and helping to resynchronize individuals who have difficulty adapting to night-shift work. have effects on circadian rhythm and sleep processes. The presence of a pharmacologically specific receptor for melatonin in which the molecular structure is known are referred to as MT1, MT2, and MT3 receptors. The MT1 and MT2 receptors are high-affinity G protein coupled receptors, whereas MT3 is a form of quinone reductase. The MT1 receptor appears to be primarily involved in initiating sleep, whereas the MT2 receptor appears to mediate melatonins effect in the eye, circadian rhythm, and vascular effects. The importance of MT3, although widely distributed in different tissues, is currently unknown. The normal physiological concentration of melatonin observed at night is approximately 100 to 200 pg/mL, and oral doses of 0.1 to 0.3 mg of melatonin are adequate to obtain these concentrations even though melatonin frequently is given at doses of 1 to 10 mg to obtain supraphysiological levels. These higher doses may be the reason for some of the side effects not currently associated with the melatonin receptors.
Veterinary Drugs and Treatments
Melatonin may be useful to treat Alopecia-X in Nordic breeds, canine
pattern baldness, or canine recurrent flank alopecia in dogs.
It has been used anecdotally for the treatment of sleep cycle disorders
in cats and geriatric dogs and to treat phobias and separation
anxiety in dogs. Melatonin implants are used in the mink and fox
pelt industries to promote the development of luxurious hair coats.
Implants are also used to improve early breeding and ovulation
rates in sheep and goats. Preliminary research is being done for this
purpose in horses also.
In pigs, one study (Bubenik, Ayles et al. 1998) demonstrated that
5 mg/kg in feed reduced the incidence
of gastric ulcers in young
pigs.
Metabolism
Hepatically metabolized to at least 14 identified metabolites (identified in mouse urine): 6-hydroxymelatonin glucuronide, 6-hydroxymelatonin sulfate, N-acetylserotonin glucuronide, N-acetylserotonin sulfate, 6-hydroxymelatonin, 2-oxomelatonin, 3-hydroxymelatonin, melatonin glucuronide, cyclic melatonin, cyclic N-acetylserotonin glucuronide, cyclic 6-hydroxymelatonin, 5-hydroxyindole-3-acetaldehyde, di-hydroxymelatonin and its glucuronide conjugate. 6-Hydroxymelatonin glucuronide is the major metabolite found in mouse urine (65-88% of total melatonin metabolites in urine).
storage
Store at -20°C
Properties of Melatonine
| Melting point: | 116.5-118 °C (lit.) |
| Boiling point: | 374.44°C (rough estimate) |
| Density | 1.1099 (rough estimate) |
| refractive index | 1.6450 (estimate) |
| Flash point: | 9℃ |
| storage temp. | -20°C |
| solubility | Soluble in ethanol to at least 50mg/ml |
| form | Powder |
| pka | 16.26±0.46(Predicted) |
| color | white to off-white |
| Merck | 14,5816 |
| BRN | 205542 |
| CAS DataBase Reference | 73-31-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Melatonin(73-31-4) |
Safety information for Melatonine
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H370:Specific target organ toxicity, single exposure |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P311:Call a POISON CENTER or doctor/physician. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Melatonine
| InChIKey | DRLFMBDRBRZALE-UHFFFAOYSA-N |
Melatonine manufacturer
Chemixl intermediates pvt Ltd
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran


![ACETYL-5'-METHOXYTRYPTAMINE, N-[METHOXY-14C]](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB5209354.gif)
You may like
-
 Melatonin 99%View Details
Melatonin 99%View Details -
 MELATONIN 99%View Details
MELATONIN 99%View Details -
 Melatonin 73-31-4 99%View Details
Melatonin 73-31-4 99%View Details
73-31-4 -
 73-31-4 95-99%View Details
73-31-4 95-99%View Details
73-31-4 -
 Melatonin CAS 73-31-4View Details
Melatonin CAS 73-31-4View Details
73-31-4 -
 Melatonin 98%View Details
Melatonin 98%View Details -
 Melatonin extrapure CAS 73-31-4View Details
Melatonin extrapure CAS 73-31-4View Details
73-31-4 -
 Melatonin 97% CAS 73-31-4View Details
Melatonin 97% CAS 73-31-4View Details
73-31-4
