Manganese carbonate
Synonym(s):Manganese carbonate;Manganese(2+) carbonate;Manganous carbonate
- CAS NO.:598-62-9
- Empirical Formula: CMnO3
- Molecular Weight: 114.95
- MDL number: MFCD00011116
- EINECS: 209-942-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
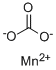
What is Manganese carbonate?
Chemical properties
Brown powder
The Uses of Manganese carbonate
Manganese carbonate (MnCO3) is used in the production of iron ore and as a chemical reagent.
The Uses of Manganese carbonate
Manganese(II) carbonate occurs in nature as the mineral rhodochrosite [14476-12-1] (manganese spar). This ore also is used to produce manganese dioxide (by electrolytic process). The pure compound is used as gemstones; and as a pigment (manganese white).
The Uses of Manganese carbonate
As pigment"manganese white"; drier for varnishes; in feeds.
Preparation
Manganese(II) carbonate is mined from its naturally occurring mineral rhodochrosite. The compound may be prepared in the laboratory as a palepink precipitate by adding sodium bicarbonate to a solution of manganese(II) salt saturated with carbon dioxide. The product obtained is monohydrate, MnCO3•H2O. However, if the carbon dioxide-saturated solution, together with the above monohydrate precipitate, is heated in the absence of atmosphere oxygen, the monohydrate MnCO3•H2O is converted into the anhydrous MnCO3.
What are the applications of Application
Manganese carbonate is extensively utilized as an additive to plant fertilizers to treat manganese deficient crops. It is also employed in health foods, in ceramics as a glaze colorant and flux, and in concrete stains. It is utilized in medicine as a hematinic (a nutrient required for the formation of blood cells in the process of haematopoiesis. The main hematinics are iron, B12, and folate).
General Description
Manganese(II) carbonate is a chemical compound that has a structure similar to calcite, with octahedral co-ordination symmetry. It is a carbonate that is insoluble in water and on treatment with acid it gives water soluble salts. It is a widely used material in plant fertilization as an additive that cures the magnesium deficiency in crops.
Flammability and Explosibility
Non flammable
Properties of Manganese carbonate
| Melting point: | 350°C (dec.) |
| Density | 3.12 g/mL at 25 °C(lit.) |
| solubility | dilute aqueous acid: slightly soluble(lit.) |
| form | Powder |
| color | Light brown to violet |
| Specific Gravity | 3.125 |
| Water Solubility | Soluble in water(0.065g/L), dilute inorganic acids. Insoluble alcohol. |
| Merck | 14,5726 |
| Solubility Product Constant (Ksp) | pKsp: 10.63 |
| Exposure limits | ACGIH: TWA 0.02 mg/m3; TWA 0.1 mg/m3 OSHA: Ceiling 5 mg/m3 NIOSH: IDLH 500 mg/m3; TWA 1 mg/m3; STEL 3 mg/m3 |
| Stability: | Stable. Incompatible with strong acids, strong oxidizing agents. May be moisture senstive. |
| CAS DataBase Reference | 598-62-9(CAS DataBase Reference) |
| EPA Substance Registry System | Manganese carbonate (1:1) (598-62-9) |
Safety information for Manganese carbonate
Computed Descriptors for Manganese carbonate
Manganese carbonate manufacturer
Ultra Chemical Works
Kronox Lab Sciences Pvt Ltd
Cosmic Chemicals
Kezi Industries (Sam Industries)
Monopoly Innovations Pvt. Ltd.
Anand Agencies
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Manganese Carbonate 98%View Details
Manganese Carbonate 98%View Details -
 Manganese Carbonate 99%View Details
Manganese Carbonate 99%View Details -
 Manganese(II) carbonate CAS 598-62-9View Details
Manganese(II) carbonate CAS 598-62-9View Details
598-62-9 -
 Manganese(II) carbonate CAS 598-62-9View Details
Manganese(II) carbonate CAS 598-62-9View Details
598-62-9 -
 Manganese(II) carbonate CAS 598-62-9View Details
Manganese(II) carbonate CAS 598-62-9View Details
598-62-9 -
 Manganese(II) carbonate 98%View Details
Manganese(II) carbonate 98%View Details -
 Manganese(II) carbonate CAS 598-62-9View Details
Manganese(II) carbonate CAS 598-62-9View Details
598-62-9 -
 Manganese(II) carbonate CAS 598-62-9View Details
Manganese(II) carbonate CAS 598-62-9View Details
598-62-9