Ethylene carbonate
Synonym(s):1,3-Dioxolan-2-one;1,3-Dioxolane-2-one;EC;Ethylene carbonate;Glycol carbonate
- CAS NO.:96-49-1
- Empirical Formula: C3H4O3
- Molecular Weight: 88.06
- MDL number: MFCD00005382
- EINECS: 202-510-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
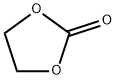
What is Ethylene carbonate?
Chemical properties
colourless crystals
The Uses of Ethylene carbonate
Ethylene carbonate is an ester of ethylene glycol and carbonic acid. Ethylene carbonate is used as a polar solvent with a molecular dipole moment of 4.9 D, only 0.1 D lower than that of propylene carb onate. It can be used as a high permittivity component of electrolytes in lithium batteries.
The Uses of Ethylene carbonate
Solvent for many polymers and resins, plasticisers, rubbers and textiles.Ethylene carbonate is used as a polar solvent and a high permittivity component of electrolytes in lithium batteries. It finds application in surface coatings, dyes, fibers and plastics. It is also used as a plasticizer and as a precursor to vinylene carbonate. Further, it is used in the preparation of dimethyl carbonate, which acts as a useful solvent and a mild methylating agent.
The Uses of Ethylene carbonate
Ethylene carbonate has been used:
In the synthesis of aliphatic polyurethanes using diamines and diols.
As a precursor for ring-opening polymerization using KOH as initiator.
As a reactant in the preparation of dimethyl carbonate, glycerol carbonate by transesterification reaction.
Definition
ChEBI: Ethylene carbonate is a carbonate ester.
Flammability and Explosibility
Non flammable
Purification Methods
Dry 1,3-dioxolan-2-one over P2O5, then fractionally distil it at low or atmospheric pressure. Recrystallise it from dry Et2O (plates, m 36.5o, 38.5-40o was also reported). It is soluble in H2O. [Beilstein 19 II 135,19 III/IV 1556, 19/4 V 6.]
Properties of Ethylene carbonate
| Melting point: | 35-38 °C(lit.) |
| Boiling point: | 243-244 °C740 mm Hg(lit.) |
| Density | 1.321 g/mL at 25 °C(lit.) |
| vapor density | 3.04 (vs air) |
| vapor pressure | 0.02 mm Hg ( 36.4 °C) |
| refractive index | 1.4199 |
| Flash point: | 320 °F |
| storage temp. | Store below +30°C. |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | Crystalline Low Melting Solid |
| pka | 3.86[at 20 ℃] |
| Specific Gravity | 1.321 |
| color | White to yellow |
| PH | 7 (200g/l, H2O, 20℃) |
| Odor | odorless |
| explosive limit | 3.6-16.1%(V) |
| Water Solubility | 214 g/L (20 ºC) |
| BRN | 106249 |
| Dielectric constant | 69.4(91℃) |
| Stability: | Stable. Incompatible with strong oxidizing agents, acids, bases, reducing agents. |
| CAS DataBase Reference | 96-49-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Ethylene carbonate(96-49-1) |
| EPA Substance Registry System | Ethylene carbonate (96-49-1) |
Safety information for Ethylene carbonate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H319:Serious eye damage/eye irritation H373:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P314:Get medical advice/attention if you feel unwell. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Ethylene carbonate
| InChIKey | KMTRUDSVKNLOMY-UHFFFAOYSA-N |
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran
You may like
-
 96-49-1 Ethylene Carbonate 98%View Details
96-49-1 Ethylene Carbonate 98%View Details
96-49-1 -
 Ethylene carbonate 99% (GC) CAS 96-49-1View Details
Ethylene carbonate 99% (GC) CAS 96-49-1View Details
96-49-1 -
 Ethylene carbonate 98% CAS 96-49-1View Details
Ethylene carbonate 98% CAS 96-49-1View Details
96-49-1 -
 Ethylene carbonate, 98% CAS 96-49-1View Details
Ethylene carbonate, 98% CAS 96-49-1View Details
96-49-1 -
 Ethylene Carbonate CAS 96-49-1View Details
Ethylene Carbonate CAS 96-49-1View Details
96-49-1 -
 Ethylene Carbonate CASView Details
Ethylene Carbonate CASView Details -
 Ethylene carbonate CAS 96-49-1View Details
Ethylene carbonate CAS 96-49-1View Details
96-49-1 -
 ETHYLENE CARBONATE CAS 96-49-1View Details
ETHYLENE CARBONATE CAS 96-49-1View Details
96-49-1
