Lithium hydroxide monohydrate
Synonym(s):Lithine hydrate;Lithium Hydroxide;Lithium hydroxide hydrate;Lithium Hydroxide, Monohydrate
- CAS NO.:1310-66-3
- Empirical Formula: Li.HO.H2O
- Molecular Weight: 41.96
- MDL number: MFCD00149772
- EINECS: 603-454-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-03-28 15:28:31
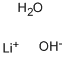
What is Lithium hydroxide monohydrate?
The Uses of Lithium hydroxide monohydrate
Lithium hydroxide monohydrate is used for the production of lithium greases, lithium soaps, lithium stearate and lithium salts. It finds application as a carbon dioxide adsorbent in breathing gas purification systems for spacecrafts, submarines and rebreathers; as a storage-battery electrolyte; as a heat transfer medium and as a catalyst for polymerization reaction. It is also used in ceramics and some portland cement formulations.
The Uses of Lithium hydroxide monohydrate
Used in catalyst for alkyd resins.
What are the applications of Application
Lithium hydroxide monohydrate is a compound used in the preparation of lithium salts
Preparation
Lithium hydroxide monohydrate may also be made by the evaporation of a solution prepared by the reaction of calcium hydroxide and lithium carbonate.Anhydrous lithium hydroxide may be prepared by heating the monohydrate in air or in a vacuum.
General Description
Small colorless crystals. Denser than water. Contact may cause severe irritation to skin, eyes, and mucous membranes. Toxic by ingestion, inhalation and skin absorption. Used to make electric storage batteries, soaps, and lubricants.
An aqueous solution of a lithium salt below its boiling point has been considered for both a tritium breeding blanket and as a coolant in several fusion reactor designs. In order to breed sufficient tritium to fuel the reactor, a high concentration of lithium is desirable and lithium hydroxide is sufficiently soluble for this purpose. The solution would be contained in pipes and heat exchangers, most probably made from carbon steel.
Air & Water Reactions
Soluble in water; heat of dissolution may generate steam and cause spattering.
Reactivity Profile
LITHIUM HYDROXIDE MONOHYDRATE neutralizes acids exothermically to form salts plus water. Reacts with certain metals (such as aluminum and zinc) to form oxides or hydroxides of the metal and generate gaseous hydrogen. May initiate polymerization reactions in polymerizable organic compounds, especially epoxides. May generate flammable and/or toxic gases with ammonium salts, nitrides, halogenated organics, various metals, peroxides, and hydroperoxides. May serve as a catalyst. Reacts when heated above about 84°C with aqueous solutions of reducing sugars other than sucrose to evolve toxic levels of carbon monoxide [Bretherick, 5th Ed., 1995].
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Purification Methods
It crystallises from hot water (3mL/g) as the monohydrate. It is dehydrated at 150o in a stream of CO2-free air. It sublimes at 220o with partial decomposition [Cohen Inorg Synth V 3 1957, Bravo Inorg Synth VII 1 1963].
Properties of Lithium hydroxide monohydrate
| Melting point: | 462 °C |
| Boiling point: | 920 °C |
| Density | 1.51 |
| storage temp. | Store at room temperature. |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | Solid |
| color | White to cream or yellow |
| Specific Gravity | 1.51 |
| PH Range | 14 |
| PH | ~12 (25℃, 1M in H2O) |
| Odor | Odorless |
| Water Solubility | 109 g/L (20 ºC) |
| Sensitive | Air Sensitive & Hygroscopic |
| λmax | λ: 260 nm Amax: 0.02 λ: 280 nm Amax: 0.02 |
| Merck | 14,5534 |
| Stability: | Stable. Incompatible with moisture. strong acids, carbon dioxide. Absorbs carbon dioxide from the air. |
| CAS DataBase Reference | 1310-66-3(CAS DataBase Reference) |
| NIST Chemistry Reference | LiOH(1310-66-3) |
| EPA Substance Registry System | Lithium hydroxide (Li(OH)), monohydrate (1310-66-3) |
Safety information for Lithium hydroxide monohydrate
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Lithium hydroxide monohydrate
| InChIKey | WMFOQBRAJBCJND-UHFFFAOYSA-M |
Lithium hydroxide monohydrate manufacturer
JSK Chemicals
ARRAKIS INDUSTRIES LLP
ASM Organics
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran
You may like
-
 Lithium hydroxide monohydrate CAS 1310-66-3View Details
Lithium hydroxide monohydrate CAS 1310-66-3View Details
1310-66-3 -
 Lithium hydroxide monohydrate CAS 1310-66-3View Details
Lithium hydroxide monohydrate CAS 1310-66-3View Details
1310-66-3 -
 Lithium hydroxide monohydrate CAS 1310-66-3View Details
Lithium hydroxide monohydrate CAS 1310-66-3View Details
1310-66-3 -
 Lithium hydroxide monohydrate CAS 1310-66-3View Details
Lithium hydroxide monohydrate CAS 1310-66-3View Details
1310-66-3 -
 Lithium hydroxide monohydrate CAS 1310-66-3View Details
Lithium hydroxide monohydrate CAS 1310-66-3View Details
1310-66-3 -
 Lithium hydroxide monohydrate CAS 1310-66-3View Details
Lithium hydroxide monohydrate CAS 1310-66-3View Details
1310-66-3 -
 Lithium Hydroxide Monohydrate pure CAS 1310-66-3View Details
Lithium Hydroxide Monohydrate pure CAS 1310-66-3View Details
1310-66-3 -
 LITHIUM HYDROXIDE MONOHYDRATE CASView Details
LITHIUM HYDROXIDE MONOHYDRATE CASView Details
