Linalool
Synonym(s):(±)-3,7-Dimethyl-1,6-octadien-3-ol;(±)-3,7-Dimethyl-3-hydroxy-1,6-octadiene;3,7-Dimethyl-1,6-octadien-3-ol;Linalool
- CAS NO.:78-70-6
- Empirical Formula: C10H18O
- Molecular Weight: 154.25
- MDL number: MFCD00008906
- EINECS: 201-134-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-19 17:28:17
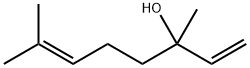
What is Linalool?
Description
Linalool has a typical floral odor free from camphoraceous and
terpenic notes.1 Synthetic linalool exhibits a cleaner and fresher
note than the natural product. It can be prepared synthetically
starting from myrcene or from dehydrolinalool.
The optically active forms (d- and ι-) and the optically inactive
form occur naturally in more than 2 0 0 oils from herbs, leaves,
flowers, and wood; the ι-form is present in the largest amounts
(80 - 85%) in the distillates from leaves of Cinnamomum cam phora var. orientalis and Cinnamomum camphora var. occidentalis
and in the distillate from Cajenne rosewood; it also has been
reported in: champaca, ylang-ylang, neroli, Mexican linaloe, ber gamot, lavandin, and others; a mixture of d- and ι-linalool has
been reported in Brazil rosewood (85%); the d-form has been
found in palmarosa, mace, sweet orange-flower distillate, petit grain, coriander (60 - 70%), marjoram, Orthodon linalooliferum
(80%), and others; the inactive form has been reported in clary
sage, jasmine, and Nectandra elaiophora.
Description
A colorless liquid with a woody, floral scent reminiscent of lavender and lily of the valley, linalool intoxicates the olfactory senses in a wide range of perfumery applications and fine cosmetics. It''s even found in Fido''s bedding, giving him the soothing odor he deserves.
Chemical properties
liquid
Chemical properties
Linalool occurs as one of its
enantiomers in many essential oils, where it is often the main component. (3R)-
(?)-Linalool, for example, occurs at a concentration of 80–85% in Ho
oils from Cinnamomum camphora; rosewood oil contains about 80%. (3S)-(+)-
Linalool makes up 60–70% of coriander oil (“coriandrol”).
Linalool is used frequently in perfumery for fruity notes and for many
floral fragrance compositions (lily of the valley, lavender, and neroli). Because of its
relatively high volatility, it imparts naturalness to top notes. Since linalool is stable
in alkali, it can be used in soaps and detergents. Linalyl esters can be prepared from
linalool.Most of the manufactured linalool is used in the production of vitamin E.
Chemical properties
Linalool has a typical pleasant floral odor, free from camphoraceous and terpenic notes. Synthetic linalool exhibits a cleaner and fresher note than the natural products.
Physical properties
Properties. Racemic linalool is, similarly to the individual enantiomers, a colorless liquid with a floral, fresh odor, reminiscent of lily of the valley. However, the enantiomers differ slightly in odor. Together with its esters, linalool is one of the most frequently used fragrance substances and is produced in large quantities. In the presence of acids, linalool isomerizes readily to geraniol, nerol, and α-terpineol. It is oxidized to citral, for example, by chromic acid. Oxidation with peracetic acid yields linalool oxides, which occur in small amounts in essential oils and are also used in perfumery. Hydrogenation of linalool gives tetrahydrolinalool, a stable fragrance material. Its odor is not as strong as, but is fresher than, that of linalool. Linalool can be converted into linalyl acetate by reaction with ketene or with an excess of boiling acetic anhydride.
Occurrence
The optically active forms (d- and l-) and the optically inactive form occur naturally in more than 200 oils from herbs, leaves, flowers and wood; the l-form is present in the largest amounts (80 to 85%) in the distillates from leaves of Cinnamomum camphora var. orientalis and Cinnamomum camphora var. occidentalis and in the distillate from Cajenne rosewood; it also has been reported in champaca, ylang-ylang, neroli, Mexican linaloe, bergamot and lavandin; a mixture of d- and l-linalool has been reported in Brazil rosewood (85%); the d-form has been found in palmarosa, mace, sweet orange-flower distillate, petitgrain, coriander (60 to 70%), marjoram and Orthodon linalooliferum (80%); the inactive form has been reported in clary sage, jasmine and Nectandra elaiophora. Also reported found in over 280 products including apple, citrus peel oils and juices, berries, grapes, guava, celery, peas, potato, tomato, cinnamon, cloves, cassia, cumin, ginger, mentha oils, mustard, nutmeg, pepper, thymus, cheeses, grape wines, butter, milk, rum, cider, tea, passion fruit, olive, mango, beans, coriander, cardamom and rice.
The Uses of Linalool
Linalool is one of the allergens of Ylang-Ylang oil (extract from Cananga odorata).
The Uses of Linalool
linalool is a fragrant component of both lavender and coriander. It can be incorporated into cosmetics for perfuming, deodorant, or odor-masking activity.
The Uses of Linalool
perfume use
Definition
ChEBI: A monoterpenoid that is octa-1,6-diene substituted by methyl groups at positions 3 and 7 and a hydroxy group at position 3. It has been isolated from plants like Ocimum canum.
Preparation
In the 1950s, nearly all linalool used in perfumery was isolated from
essential oils, particularly from rosewood oil. Currently, this method no longer
plays a commercial role.
Since linalool is an important intermediate in the manufacture of vitamin E,
several large-scale processes have been developed for its production. Preferred
starting materials and/or intermediates are the pinenes and 6-methyl-5-hepten-
2-one. Most perfumery-grade linalool is synthetic.
1) Isolation from essential oils: Linalool can be isolated by fractional distillation
of essential oils, for example, rosewood oil and coriander oil, of which Brazilian
rosewood oil was the most important.
2) Synthesis from α-pinene: α-Pinene from turpentine oil is selectively hydrogenated
to cis-pinane, which is oxidized with oxygen in the presence of
a radical initiator to give a mixture of about 75% cis-pinane and 25% transpinane
hydroperoxide.The mixture is reduced to the corresponding pinanols
either with sodium bisulfite (NaHSO3) or with a catalyst. The pinanols can
be separated by fractional distillation and are pyrolyzed to linalool: (?)-α-
pinene yields cis-pinanol and (+)-linalool, whereas (?)-linalool is obtained
from trans-pinanol.
3) Synthesis from ??-pinene: For a description of this route, see under Geraniol.
Addition of hydrogen chloride to myrcene (obtained from β-pinene) results in a mixture of geranyl, neryl, and linalyl chlorides. Reaction of this mixture
with acetic acid–sodium acetate in the presence of copper(I) chloride gives
linalyl acetate in 75–80% yield. Linalool is obtained after saponification.
4) Synthesis from 6-methyl-5-hepten-2-one:The total synthesis of linalool starts
with 6-methyl-5-hepten-2-one; several large-scale processes have been developed
for synthesizing this compound:
a. Addition of acetylene to acetone results in the formation of 2-methyl-3-
butyn-2-ol, which is hydrogenated to 2-methyl-3-buten-2-ol in the presence
of a palladium catalyst.This product is converted into its acetoacetate
derivative with diketene or with ethyl acetoacetate. The acetoacetate
undergoes rearrangement when heated (Carroll reaction) to give
6-methyl-5-hepten-2-one:
b. In another process, 6-methyl-5-hepten-2-one is obtained by reaction of
2-methyl-3-buten-2-ol with isopropenyl methyl ether followed by a
Claisen rearrangement:
c. A third synthesis starts fromisoprene, which is converted into 3-methyl-2-
butenyl chloride by addition of hydrogen chloride. Reaction of the chloride
with acetone in the presence of a catalytic amount of an organic base
leads to 6-methyl-5-hepten-2-one:
d. In another process, 6-methyl-5-hepten-2-one is obtained by isomerization
of 6-methyl-6-hepten-2-one.The latter can be prepared in two steps
from isobutylene and formaldehyde. 3-Methyl-3-buten-l-ol is formed in
the first step and is converted into 6-methyl-6-hepten-2-one by
reaction with acetone. 6-Methyl-5-hepten-2-one is converted into linalool in excellent yield by base-catalyzed ethynylation with acetylene
to dehydrolinalool. This is followed by selective hydrogenation of
the triple bond to a double bond in the presence of a palladium carbon
catalyst.
Aroma threshold values
Detection: 4 to 10 ppb
Taste threshold values
Taste characteristics at 5 ppm: green, apple and pear with an oily, waxy, slightly citrus note.
Synthesis Reference(s)
Tetrahedron, 33, p. 579, 1977 DOI: 10.1016/S0040-4020(77)80019-7
General Description
Linalool is a monoterepene compound. It is the major component of many essential oils. Anti-inflammatory properties of (?) linalool, a naturally occurring enantiomer, has been studied. It is also the major constituent of the basil and thyme essential oil and its anti-microbial effect was determined using the agar well diffusion assay. Linalool is reported to have dose-dependent marked sedative effects at the central nervous system (CNS), including hypnotic, anticonvulsant and hypothermic properties. Linalool is reported to have a lemon like odor.
Contact allergens
Linalool is a terpene chief constituent of linaloe oil,also found in oils of Ceylon cinnamon, sassafras,orange flower, bergamot, Artemisia balchanorum, ylang-ylang. This frequently used scented substance is a sensitizer by the way of primary or secondary oxida-tion products. As a fragrance allergen, linalool has to be mentioned by name in cosmetics within the EU
Pharmacology
l-Linalool showed no sedative action in the mouse motility test when injected
ip at a dose of 100 mg/kg (Binet, Binet, Miocque, Roux & Bernier, 1972). but Wagner & Sprinkmeyer
(1973) reported that linalool depressed spontaneous motility of mice at doses of 31-6 and 100 mg/kg.
Linalool showed spasmolytic action against carbachol-, histamine- and barium chloride-induced
contractions in isolated guinea-pig ileum, the ED50 being about 100-200 mg/litre (Wagner & Sprinkmeyer.
1973). and in the isolated rat duodenum, contractions caused by 0.05 /ig acetylcholine/ml
were inhibited by 50% by 10 μg l-linalool/ml (Binet et al. 1972). Linalool showed slight papavarinelike
and very slight atropine-like antispasmodic action on small intestine isolated from the mouse
(Imaseki & Kitabatake, 1962).
In studies carried out by Atanassova-Shopova et al. (1973), the ED50 for preventing tonic hyperextension
of the hind limbs of rats from electric shock was found to be 135 mg/kg given ip. Linalool
had a marked anticonvulsive and protective effect on pentylenetetrazol convulsions in mice at 150,175 and 200 mg/kg and in rats at 200 and 300 mg/kg. It showed a slight antistrychnine effect
in mice at high and toxic doses (300 mg/kg), reduced motor activity of mice at 100 mg/kg, and
at 50 mg/kg slightly decreased the motor activity of amphetamine- or caffeine-stimulated mice.
The TD50 (neurotoxic dose) of linalool for influencing motor co-ordination of mice in the Rota-rod
test was found to be 178 mg/kg. Linalool at doses of 50 or 100 mg/kg prolonged the narcotic effects
of hexobarbitone, alcohol and chloral hydrate.
The equilibrium and spontaneous or reflex activity of the goldfish, Carassium auratus, was disturbed
by exposure to aquarium water containing a 0-13 ml/litre concentration of a suspension
containing 1 ml linalool plus 9 ml of a 10% aqueous solution of Tween 80, and the agressiveness
of the male fighting fish, Betta splendens, was only very slightly inhibited by exposure to aquarium
water containing 0-3 ml of the same suspension of linalool/litre (Binet, 1972).
Linalool and other terpene alcohols were found to be useful in man as sedatives and spasmolytics
when administered in doses of 0.01-1 g, the effects having been tested in mice, goldfish, and rats
(Laboratoires Meram, 1966).
Linalool depressed frog-heart activity in doses above 0-2 mg/g (Lysenko, 1962). Vasodilation by
direct action of linalool upon the blood vessels was demonstrated by Northover & Verghese (1962).
An iv dose of 9.2 mg/kg was required to produce a 25% fall in systolic arterial blood pressure
in the anaesthetized dog and a hypotensive response was also observed in the decerebrated and
despinalized dog. A dose of 0.05 g in fluid perfusing the leg of an anaesthetized dog or the isolated
ear of a rabbit produced a maximum increase of 120% or 90%, respectively, in venous outflow
over pre-injection values. Linalool dilated the small blood vessels of the exposed mesorchium of
the anaesthetized mouse, lowering the threshold for electronic stimulation. Incubation of human,
bovine and canine aortae in 015 M-linalool failed to stabilize the structure of the aortic wall proteins
against hydrothermal shrinkage (Milch, 1965). Linalool inhibited incorporation of acetic acid or
mevalonic acid into total or digitonin-precipitable nonsaponifiable lipids by rat-liver homogenates
(Gey, Pletscher, Isler, Riiegg, Saucy & Wiirsch, 1960).
Anticancer Research
Studies of antitumor activities and toxicity were done on solid S-180 tumor-bearingSwiss albino mice. It results in an induction of oxidative stress with an antitumoractivities result. In comparison with cyclophosphamide, antioxidant effects wereobserved in the liver and modulation of proliferation of spleen cells in tumor-bearingmice challenged with lipopolysaccharides, while both were seriously affected bycyclophosphamide (Costa et al. 2015).
Synthesis
It can be prepared synthetically starting from myrcene or from dehydrolinalool; it can be obtained by fractional distilla tion and subsequent rectification from the oils of Cajenne rosewood (Licasia guaianensis, Ocotea caudata), Brazil rosewood (Ocotea parviflora), Mexican linaloe, shiu (Cinnamomum camphora Sieb. var. linalooifera) and coriander seeds (Coriandrum sativum L.).
Metabolism
The metabolism of 14C-labelled linalool in the rat was studied by Parke, Rahman & Walker (1974a). An intragastric dose of 500 mg linalool/kg body weight was largely (93%) excreted within 72 hr in the urine (55%), faeces (23%) and expired air (15%). The radioactivity remaining after 72 hr was located mainly in the liver (0-5%), gut (0-6%), skin (0-8%) and skeletal muscle (1-2%). Rapid urinary excretion indicated that linalool was rapidly absorbed from the gut, while delay in excretion in the expired air suggested that linalool might enter intermediary metabolism and also be metabolized by conjugation in the bile and urine. Ip administration of 20 mg linalool indicated that enterohepatic circulation occurred, resulting in a short-term metabolic load on the liver and delayed faecal excretion. The metabolism of large doses in the rat, with rapid excretion of linalool and its metabolites, suggests no long-term hazard from tissue accumulation on chronic exposure to concentrations normally encountered in foods, although enterohepatic circulation might prolong the metabolic load on the liver over a relatively short period. A study of the effects of linalool and other terpenoids on hepatic drug-metabolizing enzymes suggested that these compounds induce the enzymes involved in their own metabolism. Linalool, which is metabolized by reduction and conjugation with glucuronic acid, increased the activity of 4-nitrobenzoate reductase but did not increase other enzymes studied (Parke & Rahman, 1969). Linalool can be metabolized by micro- organisms. l-Linalool was partially oxidized by incubation with Aspergillus nig er (Goto, 1967). The linalool content of grape essential oil decreased during must fermentation and wine formation (Rodopulo, Egorov, Bezzubov, Kormakova & Megrelidze, 1972). A strain of Pseudomonas pseudomallei, isolated from soil, metabolized linalool with the formation of camphor, 4-methyl-4-vinylbutyrolactone, 4-methyl- trans-3-hexenoic acid, and 2,6-dimethyl-6- hydroxy- trans-2,7-octadienoic acid (Mizutani, Hayashi, Ueda & Tatsumi, 1971).
Properties of Linalool
| Melting point: | 25°C |
| Boiling point: | 194-197 °C/720 mmHg (lit.) |
| Density | 0.87 g/mL at 25 °C (lit.) |
| vapor pressure | 0.17 mm Hg ( 25 °C) |
| refractive index | n |
| FEMA | 2635 | LINALOOL |
| Flash point: | 174 °F |
| storage temp. | 2-8°C |
| solubility | ethanol: soluble1ml/4ml, clear, colorless (60% ethanol) |
| form | Liquid |
| pka | 14.51±0.29(Predicted) |
| Specific Gravity | 0.860 (20/4℃) |
| color | Clear colorless to pale yellow |
| PH | 4.5 (1.45g/l, H2O, 25℃) |
| Odor | at 100.00 %. citrus floral sweet bois de rose woody green blueberry |
| explosive limit | 0.9-5.2%(V) |
| Water Solubility | 1.45 g/L (25 ºC) |
| Merck | 14,5495 |
| JECFA Number | 356 |
| BRN | 1721488 |
| Stability: | Stable. Incompatible with strong oxidizing agents. Combustible. |
| CAS DataBase Reference | 78-70-6(CAS DataBase Reference) |
| NIST Chemistry Reference | 2,6-Dimethylocta-2,7-dien-6-ol(78-70-6) |
| EPA Substance Registry System | 3,7-Dimethyl-1,6-octadien-3-ol (78-70-6) |
Safety information for Linalool
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P272:Contaminated work clothing should not be allowed out of the workplace. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Linalool
| InChIKey | CDOSHBSSFJOMGT-UHFFFAOYSA-N |
Linalool manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Linalool (Ex-Basil Oil) 98%View Details
Linalool (Ex-Basil Oil) 98%View Details -
 78-70-6 99%View Details
78-70-6 99%View Details
78-70-6 -
 Linalool CAS 78-70-6View Details
Linalool CAS 78-70-6View Details
78-70-6 -
 Linalool CAS 78-70-6View Details
Linalool CAS 78-70-6View Details
78-70-6 -
 Linalool 95% CAS 78-70-6View Details
Linalool 95% CAS 78-70-6View Details
78-70-6 -
 Natural Linalool (Ex Basil)View Details
Natural Linalool (Ex Basil)View Details
78-70-6 -
 LINALOOLView Details
LINALOOLView Details
78-70-6 -
 Natural Aroma Linalool 98% (Natural)View Details
Natural Aroma Linalool 98% (Natural)View Details
78-70-6
