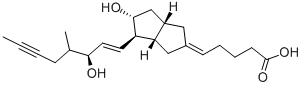
Iloprost
Synonym(s):(E)-(3aS, 4R, 5R, 6aS)-Hexahydro-5-hydroxy-4-[(E)-(3S,4RS)-3-hydroxy-4-methyl-1-octen-6-ynyl]-Δ2(1H),Δ-pentalenevaleric acid;Cilaprost;ZK 36374; (5E)-5-[(3aS,4R,5R,6aS)-Hexahydro-5-hydroxy-4-[(1E,3S)-3-hydroxy-4-methyl-1-octen-6-ynyl]-2(1H)-pentalenylidene]pentanoic acid
- CAS NO.:73873-87-7
- Empirical Formula: C22H32O4
- Molecular Weight: 360.49
- MDL number: MFCD00867145
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-11-08 17:00:29
What is Iloprost?
The Uses of Iloprost
A synthetic analogue of Prostacyclin (PGI2) used to treat pulmonary arterial hypertension (PAH), scleroderma, Raynaud''s phenomenon and ischemia. It acts through elevation of cAMP by binding to the prostacyclin receptor (IP receptor). Iloprost inhibits the ADP, thrombin, and collagen-induced aggregation of human platelets with an ED50 of about 13 nM. In whole animals, iloprost acts as a vasodilator, hypotensive, antidiuretic, and prolongs bleeding time.
The Uses of Iloprost
Treatment of pulmonary hypertension (prostacyclin analogue).
Definition
ChEBI: Iloprost is a carbobicyclic compound that is prostaglandin I2 in which the endocyclic oxygen is replaced by a methylene group and in which the (1E,3S)-3-hydroxyoct-1-en-1-yl side chain is replaced by a (3R)-3-hydroxy-4-methyloct-1-en-6-yn-1-yl group. A synthetic analogue of prostacyclin, it is used as the trometamol salt (generally by intravenous infusion) for the treatment of peripheral vascular disease and pulmonary hypertension. It has a role as a platelet aggregation inhibitor and a vasodilator agent. It is a monocarboxylic acid, a secondary alcohol and a carbobicyclic compound.
brand name
Ventavis (Schering).
General Description
This more chemically and biologically stable derivativeof prostacyclin is available as a solution (10 μg/mL)for nasal inhalation (Ventavis by Actelion) via a preciselycalibrated inhalation device for the treatment of pulmonaryarterial hypertension (PAH). Patients inhale 6 to 8 puffs ofaerosolized iloprost every 2 to 3 hours to produce a directvasodilatory effect on pulmonary blood vessels, therebydecreasing vascular resistance. Side effects of coughing,flushing, headaches, and jaw pain have been most commonlyreported.
Clinical Use
Iloprost is administered as an inhalation solution of a prostacyclin analog for the treatment of NYHA class III and class IV PAH. The drug also can be administered as an IV infusion. It is stable at room temperature and to light, with a body half-life of 30 minutes. Iloprost has approximately 10 times greater potency than prostacyclin as a vasodilator of the pulmonary blood vessels; this greater potency of inhaled iloprost results from coating of the drug on the alveoli of the lungs.
Side Effects
Studies have reported minor side effects, such as coughing, headaches, and jaw pain.
Drug interactions
Potentially hazardous interactions with other drugs
Anticoagulants: enhanced anticoagulant effect and
increased risk of bleeding with heparin, coumarins
and phenindione, as iloprost inhibits platelet
aggregation.
Increased risk of bleeding with NSAIDs, aspirin,
clopidogrel, eptifibatide and tirofiban.
Metabolism
On intravenous infusion iloprost is rapidly cleared from the plasma by oxidation. About 80% of the metabolites are excreted in urine and 20% in the bile.
Properties of Iloprost
| storage temp. | Amber Vial, -20°C Freezer, Under inert atmosphere |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| color | Colourless to Pale Yellow Oil to |
| Stability: | Light Sensitive |
Safety information for Iloprost
Computed Descriptors for Iloprost
Abamectin manufacturer
BDR Pharmaceuticals International Pvt Ltd
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

You may like
-
 78919-13-8 / 73873-87-7 Iloprost 98%View Details
78919-13-8 / 73873-87-7 Iloprost 98%View Details
78919-13-8 / 73873-87-7 -
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4